Abstract
Treatments for pure ground-glass nodules (GGNs) include limited resection; however, surgery is not always possible in patients with limited pulmonary functional reserve. In such patients, cryoablation may be a suitable alternative to treat a pure GGN. Here, we report our initial experience with cryoablation of a pure GGN that remained after repeated surgical resection in a patient with multiple GGNs. A 5-mm-sized pure GGN in the left lower lobe was cryoablated successfully without recurrence at the 6-month follow-up.
A pure ground-glass nodule (GGN) in the lung may represent an early lung malignancy, such as adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) (1). Approximately 20-30% of patients with adenocarcinoma and a predominantly pure GGN who undergo surgery have additional GGN lesions, either in the same or different lung lobes (2). In such cases, multiple limited resection may be an appropriate treatment if a few pure GGN lesions are scattered in multiple lobes or peripherally in one lobe, and the patient has sufficient pulmonary functional reserve (3). However, alternative options should be considered if several GGN lesions are scattered in both lungs with multiple lobes involved, and there is a risk for insufficient pulmonary functional reserve after resection. Chemotherapy has been suggested as an additional therapeutic option, if surgery is not possible due to a lack of pulmonary functional reserve (4). Another strategy is to rely on serial computed tomography (CT) scans until the nodule develops a definite solid portion. If curative therapy is not performed, there remains a significant chance for progression (1).
Cryoablation has been explored as an option for treating metastatic lesions in patients with non-small cell lung cancer who cannot undergo surgery or chemotherapy (5). However, no clinical reports of cryoablation techniques applied to multiple GGNs in the lung have been published. Here, we describe a patient with lung cancer and a pure GGN in the left lower lung lobe that persisted after several limited resections and was successfully treated with cryoablation. Our Institutional Review Board approved this report.
A 59-year-old woman visited our hospital due to abnormal chest radiography findings on a health maintenance exam at another hospital. A chest CT scan was performed at our hospital. All pre- and post-procedural CT scans were performed using a multi-detector CT scanner (Somatom Definition Flash, Enlargen Siemens, Forchheim, Germany). The scanning parameters were: 120 kVp, 200 mAs, 0.75-mm collimation, 2-mm reconstruction intervals, smooth algorithm, and pitch of 1. Several GGNs were detected by CT scan: a 12-mm-sized, partially solid nodule in the right middle lobe (Fig. 1A), a 7-mm-sized pure GGN in the right upper lobe, and two 5-mm-sized pure GGNs in each of the lower lobes (Fig. 1B, C). The patient underwent a right middle lobectomy and wedge resection of the right upper lobe using video-assisted thoracoscopic surgery. The partially solid nodule in the right middle lobe was diagnosed as adenocarcinoma, and the pure GGN in the right upper lobe was diagnosed as a MIA. The two pure GGNs in both lower lobes remained without significant change on a 10-month follow-up CT scan. An additional wedge resection was performed for the pure GGN in the right lower lobe, and this nodule was diagnosed as AIS. After these surgeries, the patient did not have sufficient pulmonary functional reserve to tolerate any additional surgery. Thus, we decided to perform cryoablation on the pure GGN in the left lower lobe.
The patient fasted for 6 hours before the procedure, and her blood coagulation parameters were checked. We used an argon and helium gas-based system (Seednet, Galil Medical, Yokneam, Israel), a 1.5-mm 17-G cryoprobe, and a thermal sensor. The procedure was performed in a fluoroscopy room by an interventional radiologist. After placing the patient in the prone position on the table, a cross-shaped radio-opaque skin marker was applied to determine the needle insertion site under fluoroscopy. Subsequently, a C-arm cone-beam CT (CBCT) scan (DynaCT, Enlargen Siemens) was taken (Fig. 1D-F) to obtain 1.5-mm thick axial, coronal, and sagittal images. Based on these images, we measured the distance from the center of the skin marker to the target GGN. The area of the skin marker was draped, and a 1% lidocaine solution was injected into the skin down to the pleural surface. After localizing the needle tip, a CBCT scan was performed to confirm the probe insertion site. A 17-G cryoprobe (Seednet, Galil Medical) was carefully introduced into the pure GGN under fluoroscopic guidance. Then, another CBCT scan was performed to confirm the location of the probe. We performed two 7-minute freezing cycles followed by 3 minutes of thawing. We used a fluoroscope and CBCT to visualize the size of the ice ball during the procedure. We took a CT scan to confirm that the ice ball had reached sufficient size, as indicated by a low attenuation region with an envelope of ground-glass opacity and a margin of 1 cm beyond the GGN. A small pneumothorax was noted after removing the cryoprobe; however, the patient was asymptomatic with stable vital signs.
We performed a follow-up CT scan 1 day after cryoablation to assess any initial changes in the ablated lesion and procedural-related side effects (Fig. 1G). The scan showed a 3-cm sized ablated zone in the left lower lobe with no major procedure-related complications, such as hemothorax. The size of the ablated zone had decreased markedly on a follow-up CT scan 2 months later. The pure GGN in the left lower lobe was judged to have been successfully ablated without recurrence on a 6-month follow-up CT scan (Fig. 1H, I).
Our results show the potential of cryoablation as a new treatment method for GGN, particularly in patients in whom surgery is contraindicated. Some controversy exists as to whether some nodules should be resected or followed by serial CT scans. Kim et al. (6) noted that several pure GGN lesions detected in patients undergoing surgery for bronchioloalveolar carcinoma did not change in size or features during follow-up. They suggested that surgical resection should be considered in selected cases in which nodules exhibit significant changes in size or appearance during follow-up. Kuriyama et al. (7) found that all GGN lesions > 10 mm were carcinomas and insisted that these lesions should be resected rather than followed. Partially solid GGN lesions should be resected regardless of size because they represent more invasive lesions than pure GGNs. Lobectomy to treat lung cancer can limit pulmonary functional reserve. Rapicetta et al. (8) reported that forced expiratory volume in 1 second after lobectomy decreases 11%. Thus, a non-surgical alternative is needed for patients with insufficient pulmonary functional reserve or in patients in whom surgery is technically not feasible.
Minimally invasive ablation techniques, such as radiofrequency ablation (RFA) and cryoablation, may be useful adjunctive treatment options for lung cancer or metastatic lesions and preserve pulmonary function (5, 9, 10). Minimally invasive ablation techniques for lung cancer have several advantages, including selective damage, minimal morbidity and mortality, minimal loss of lung function, repeatability, low cost, excellent monitoring during treatment, less pain, and shorter hospital stay (11). Ablating lung tumors is currently used for curative treatment of primary lung cancer and metastatic lung malignancies or cytoreduction (12). Curative ablation is indicated for patients with inoperable stage I primary lung cancer. Ablation is also an option in patients with lung metastases from colorectal and renal cell carcinoma, melanoma, hepatocellular carcinoma, or primary sarcoma (13).
Although RFA is a contemporary tumor ablation technique, it may cause serious complications, such as air embolism or uncontrolled pain (9, 14). Cryoablation is a thermoablational technique that consists of alternating cycles of decreasing (freezing) and increasing (thawing) temperatures, leading to direct cellular and vascular injury. Cryoablation works by forming an ice ball, which causes increased extracellular osmolarity and, as a consequence, water diffuses from the intracellular space into the extracellular space. Cryoablation does not result in substantial collagen damage compared with that of RFA. Thus, cryoablation may be a better option for patients with extensive emphysema (10).
This report had several limitations. The targeted GGN lesions were not confirmed pathologically. However, percutaneous or surgical biopsy was difficult due to the small size and multiplicity of the GGN lesions. Multiple biopsies could lead to fatal complications, such as hemothorax. Nevertheless, localized GGNs can include invasive disease, such as AIS or MIA (1). In our case, two similar nodules in the right upper and right lower lobes were identified during surgery as MIA and AIS, respectively. Cryoablation may reduce the likelihood that a remnant GGN will progress to advanced cancer and does not require additional surgery. Another limitation was the relatively short follow-up duration. Song et al. (15) reported that pure GGNs progress slowly. Thus, long-term follow-up may be necessary to determine the outcome of cryoablation for treating pure GGNs.
In conclusion, cryoablation may be a suitable adjunctive therapeutic option for pure GGNs. Cryoablation can be easily combined with other follow-up treatment strategies and therapies. Further prospective investigations with long-term follow-up are needed.
Figures and Tables
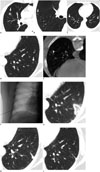
Fig. 1
59-year-old woman diagnosed with adenocarcinoma of right lung.
Computed tomography scans were obtained 1 week before surgery. A. 12-mm partially solid nodule was detected in lateral segment of right middle lobe (arrow). B. 7-mm pure ground-glass nodule (GGN) was located in right upper lobe (arrow). C. Two 5-mm GGNs were located in each of lower lobes (arrows). Computed tomography (CT) scans taken before and after cryoablation. D. Follow-up CT image after two surgeries shows pure ground-glass nodule (GGN) remaining in left lower lobe (arrow). E. Location of cryoprobe tip near GGN in left lower lobe was visualized on C-arm cone-beam CT scan. F. Projection radiography under real-time fluoroscopy shows cryoprobe introduced into lung. G. Enveloped ablated zone was noted surrounding pure GGN in left lower lobe after cryoablation. Follow-up computed tomography (CT) scan after cryoablation. H. Size of ablated zone had decreased markedly on CT scan taken 2 months after cryoablation. I. Ablated zone enveloping pure ground-glass nodule in left lower lung lobe had changed to linear parenchymal band 6 months after cryoablation.
References
1. Nakajima R, Yokose T, Kakinuma R, Nagai K, Nishiwaki Y, Ochiai A. Localized pure ground-glass opacity on high-resolution CT: histologic characteristics. J Comput Assist Tomogr. 2002; 26:323–329.
2. Lim HJ, Ahn S, Lee KS, Han J, Shim YM, Woo S, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest. 2013; 144:1291–1299.
3. Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging. 2011; 26:106–118.
4. Park JH, Lee KS, Kim JH, Shim YM, Kim J, Choi YS, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol. 2009; 10:12–20.
5. Bang HJ, Littrup PJ, Currier BP, Goodrich DJ, Aoun HD, Klein LC, et al. Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol. 2012; 23:761–769.
6. Kim HK, Choi YS, Kim J, Shim YM, Lee KS, Kim K. Management of multiple pure ground-glass opacity lesions in patients with bronchioloalveolar carcinoma. J Thorac Oncol. 2010; 5:206–210.
7. Kuriyama K, Seto M, Kasugai T, Higashiyama M, Kido S, Sawai Y, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol. 1999; 173:465–469.
8. Rapicetta C, Tenconi S, Voltolini L, Luzzi L, Scala V, Gotti G. Impact of lobectomy for non-small-cell lung cancer on respiratory function in octogenarian patients with mild to moderate chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2011; 39:555–559.
9. Hiraki T, Gobara H, Mimura H, Matsui Y, Toyooka S, Kanazawa S. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011; 142:24–30.
10. Thanos L, Mylona S, Ptohis N, Tsiouris S, Sotiropoulou E, Pomoni A, et al. Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol. 2009; 15:290–296.
11. Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010; 211:68–72.
12. Lee JM, Jin GY, Goldberg SN, Lee YC, Chung GH, Han YM, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004; 230:125–134.
13. Pereira PL, Masala S. Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol. 2012; 35:247–254.
14. Jin GY, Han YM, Lee YS, Lee YC. Radiofrequency ablation using a monopolar wet electrode for the treatment of inoperable non-small cell lung cancer: a preliminary report. Korean J Radiol. 2008; 9:140–147.
15. Song YS, Park CM, Park SJ, Lee SM, Jeon YK, Goo JM. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology. 2014; 273:276–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download