Abstract
It has been reported that the common sites of brown tumors are the jaw, pelvis, ribs, femurs and clavicles. We report our experience in a case of brown tumor of the patella caused by primary hyperparathyroidism. An initial radiograph and CT showed an osteolytic lesion and MR images showed a mixed solid and multiloculated cystic tumor in the right patella. One month after the parathyroidectomy, rapid bone formation was observed on both radiographs and CT images.
Brown tumors are focal reactive osteolytic lesions that are encountered in patients with primary or secondary hyperparathyroidism (1, 2, 3). The incidence of brown tumor has been reported to be 3% in cases of primary hyperparathyroidism and 2% in cases of secondary hyperparathyroidism (3). Brown tumors can occur in any bone, but most commonly affect the pelvis, ribs, femurs, facial bones, jaws and clavicles (3). There have been a few reports on brown tumor of the patella (4, 5, 6).
We recently treated a patient, who developed a brown tumor of the patella caused by primary hyperparathyroidism. The patients' clinical courses are discussed in this report. This report was approved by the Institutional Review Board of our institution. An informed consent was waived.
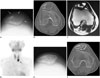
A 31-year-old female started experiencing right gonalgia without any history of antecedent trauma. The body mass index of patient was 22.0 kg/m2. The lumbar bone mineral density (BMD) measured by dual X-ray absorptiometry was 0.589 g/cm2 (T score: -3.5 standard deviation [SD]), which indicated severe osteoporosis. The range of motion of the right knee was 120° in flexion and 0° in extension. An initial radiograph showed focal loss of radiodensity in the right patella (Fig. 1A). As per multiplanar reconstruction (MPR) CT images, disruptions of the cortex around the osteolytic lesions were seen in the right patella (Fig. 1B). In addition, multiple asymptomatic osteolytic lesions were observed in the right pubis and left ilium. An axial T2-weighted MR image showed the mixed solid and multiloculated cystic nature of the tumor in the right patella (Fig. 1C). Radiologically, these well-defined osteolytic lesions were suspected to be brown tumors or giant cell tumors based on the sites of the lesions (7).
The laboratory data regarding the levels of bone metabolism markers, including serum parathyroid hormone (PTH), calcium, phosphorus, alkaline phosphatase, tartrate-resistant acid phopshatate-5b (TRACP-5b) and procollagen type 1 N propeptide (P1NP), were as follows: 1260 pg/mL (normal range, 10-65 pg/mL), 3.15 mmol/L (2.15-2.55 mmol/L), 1.8 mg/dL (2.5-4.7 mg/dL), 1320 mU/dL (120-420 mU/dL), and 855 µg/L (14.9-68.8 µg/L), respectively. Neck ultrasonography showed a hypoechoic lesion (21 × 11 mm in diameter) at the lower pole of the left lobe of the thyroid gland. A mass lesion of the parathyroid gland was orgobserved by parathyroid scintigraphy (Tc-99m 2 methoxy-isobutyl-isonitrile) (Fig. 1D).
The patient was diagnosed to have a brown tumor caused by primary hyperparathyroidism based on both the imaging and laboratory findings. A parathyroidectomy was performed by a surgical team one month after the patient's first visit to our hospital. The resected mass (20 × 15 mm in diameter) showed a smooth surface and was encapsulated. The histopathological examination of resected mass revealed parathyroid adenoma.
The serum PTH level decreased from 1260 pg/mL (before surgery) to 19 pg/mL (one day after surgery), and the serum calcium level also decreased from 3.15 mmol/L to 2.29 mmol/L, and symptoms of hypocalcemic tetany were observed soon after surgery. These findings were consistent with hungry bone syndrome (HBS) caused by parathyroidectomy. Therefore, the patient was administered high doses of calcium gluconate hydrate (5 to 100 mL/day), calcium lactate hydrate and alfacalcidol during 30 days of hospitalization. The patient reported complete resolution of pain in the right knee one month after parathyroidectomy. Rapid bone formation was observed in both radiographs and CT-MPR images (Fig. 1E, F), and the BMD increased to 0.736 g/cm2 (T score: -2.3 SD).
Brown tumors are non-neoplastic osteolytic lesions of the bone that are caused by primary or secondary hyperparathyroidism (1, 2, 3). Brown tumors can occur as solitary or multiple lesions. They may appear in any bone, but commonly affect the pelvis, ribs, femurs, humeri and other long bones, as well as the facial bones and jaws, clavicles and spine (1, 2, 3, 8, 9). Generally, brown tumors are lytic in nature with well-defined borders according to radiographs or CT scans, and similar results were seen in our case (Fig. 1A, B). The brown tumor lesions are heterogeneously hypo- and isointense to skeletal muscle on T1-weighted MR images, although the signal characteristics depend on the level of sclerosis (6).
Singh et al. (4) reported 59 cases of bone tumors of the patella. They described 46% of cases as non-neoplastic, 39% as benign, while 15% as malignant (4). Giant cell tumor is the most common disease, which affects the patella, and 19% of patella tumors have previously been reported as giant cell tumors (4). The second and third common diseases affecting the patella are chondroblastoma (15%) and aneurysmal bone cyst (10%), respectively (4). A biopsy has the potential to lead to a misdiagnosis, since brown tumor is similar to aneurysmal bone cyst or giant cell tumor in terms of the pathological tissue (10). Therefore, the imaging and laboratory findings are important for accurate differentiation of the tumor. A recent paper showed that fluoro-deoxyglucose positron emission tomography/CT is effective for detecting osteolytic lesions, such as brown tumors (11). The uptake of fluoro-deoxyglucose by osseous lesions can assist in diagnosing brown tumors with hyperparathyroidism, and abnormal uptake of fluoro-deoxyglucose can aid in differentiation of primary neoplasms (11).
Elevated levels of PTH, TRACP-5b, and P1NP were observed in the current case, which resulted in severe osteoporosis, subperiosteal bone resorption and reactive proliferation of the fibrovascular tissue in the bone marrow caused by an increase in bone turnover. In contrast, decreased levels of PTH and calcium were observed on first day subsequent to parathyroidectomy, which resulted in HBS. HBS is defined as severe and prolonged hypocalcemia and hypocalcemic symptoms after parathyroidectomy for hyperparathyroidism. Occurrence of HBS has been reported in 25-90% of cases with hyperparathyroidism with bone lesions detected radiologically, and in 0-6% of cases with hyperparathyroidism without bone lesions (12).
The patient reported complete resolution of right gonalgia after one month of resection of the tumor, and rapid and massive bone formation was observed in imaging studies. An increase in lumbar BMD was also observed. We consider that the duration of treatment in our case is much shorter than that of previous reports (13, 14).
As increased osteoclastic bone turnover results in fractures followed by influx of multinucleated macrophages, destruction of cortex and soft-tissue extension can be expected as imaging findings (3). It has been reported that the risk of pathological fracture in brown tumors correlates with the time of diagnosis (15). Therefore, it is important to diagnose or differentiate brown tumors from other tumors at an early stage, and as soon as possible.
Figures and Tables
Fig. 1
31-year-old female suffered from right knee pain.
A. Initial radiograph showed focal loss of radiodensity in right patella (arrows). B. On axial slice of CT-multiplanar reconstruction (MPR) image, disruptions of cortex around osteolytic lesions were seen in right patella. C. Axial T2-weighted MR image (repetition time/echo time = 4500/106 msec) showed mixed solid and multiloculated cystic nature of tumor in right patella. D. Mass lesion of parathyroid gland was observed by parathyroid scintigraphy (Tc-99m 2 methoxy-isobutyl-isonitrile) (arrow). E, F. One month after parathyroidectomy, bone formation and decrease in size of osteolytic lesion were observed in both radiographs (E) and CT-MPR images (F) in comparison to initial images (shown in A, B).
References
1. Takeshita T, Takeshita K, Abe S, Takami H, Imamura T, Furui S. Brown tumor with fluid-fluid levels in a patient with primary hyperparathyroidism: radiological findings. Radiat Med. 2006; 24:631–634.
2. Knowles NG, Smith DL, Outwater EK. MRI diagnosis of brown tumor based on magnetic susceptibility. J Magn Reson Imaging. 2008; 28:759–761.
3. Hong WS, Sung MS, Chun KA, Kim JY, Park SW, Lee KH, et al. Emphasis on the MR imaging findings of brown tumor: a report of five cases. Skeletal Radiol. 2011; 40:205–213.
4. Singh J, James SL, Kroon HM, Woertler K, Anderson SE, Jundt G, et al. Tumour and tumour-like lesions of the patella--a multicentre experience. Eur Radiol. 2009; 19:701–712.
5. Bhagat S, Sharma H, Bansal M, Reid R. Presentation and outcome of primary tumors of the patella. J Knee Surg. 2008; 21:212–216.
6. Vernace N, Bancroft LW. Multiple brown tumors and pathologic patellar fracture in a patient with secondary hyperparathyroidism. Orthopedics. 2014; 37:564–567.
7. Hsieh MC, Ko JY, Eng HL. Pathologic fracture of the distal femur in osteitis fibrosa cystica simulating metastatic disease. Arch Orthop Trauma Surg. 2004; 124:498–501.
8. Jouan A, Zabraniecki L, Vincent V, Poix E, Fournié B. An unusual presentation of primary hyperparathyroidism: severe hypercalcemia and multiple brown tumors. Joint Bone Spine. 2008; 75:209–211.
9. Chew FS, Huang-Hellinger F. Brown tumor. AJR Am J Roentgenol. 1993; 160:752.
10. Campanacci M. "Brown tumors" in primary hyperparathyroidism. In : Campanacci M, editor. Bone and soft tissue tumors. 2nd ed. New York: Springer-Verlag Wien;1999. p. 877–899.
11. Lacativa PG, Franco FM, Pimentel JR, Patrício Filho PJ, Goncçalves MD, Farias ML. Prevalence of radiological findings among cases of severe secondary hyperparathyroidism. Sao Paulo Med J. 2009; 127:71–77.
12. Witteveen JE, van Thiel S, Romijn JA, Hamdy NA. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013; 168:R45–R53.
13. Di Daniele N, Condò S, Ferrannini M, Bertoli M, Rovella V, Di Renzo L, et al. Brown tumour in a patient with secondary hyperparathyroidism resistant to medical therapy: case report on successful treatment after subtotal parathyroidectomy. Int J Endocrinol. 2009; 2009:827652.
14. Wang X, Wang M, Zhang J, Zhu Y, Zhu M, Gao H, et al. Humeral brown tumor as first presentation of primary hyperparathyroidism caused by ectopic parathyroid adenomas: report of two cases and review of literature. Int J Clin Exp Pathol. 2014; 7:7094–7099.
15. Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000; 321:598–602.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download