Abstract
Renal transplantation is the treatment of choice for patients with chronic renal failure, which produces a dramatic improvement in the quality of life and survival rates, in comparison to long-term dialysis. Nowadays, new imaging modalities allow early diagnosis of complications, and thanks to the recent developments of interventional techniques, surgery may be avoided in most cases. Knowledge in the types of renal transplant complications is fundamental for a correct pre-operative planning. In this article, we described the most common or clinically relevant renal transplant complications and explained their interventional management.
Renal transplantation has become the standard treatment for end-stage renal disease, which produces a drastic improvement of patient's survival, in comparison with long-term dialysis (1).
With the recent development of interventional techniques, many complications can be managed conservatively with minimally invasive percutaneous procedures, which enable to avoid open surgery or stabilize the patient's condition prior to open surgical reintervention.
The aim of this article is to describe the most common or clinically relevant surgical complications of renal transplant and to explain, when possible, their interventional management.
Renal artery stenosis (RAS) represents the most common vascular complication, with an estimated incidence between 3-23% of all renal transplant recipients (2). Main manifestation is severe hypertension refractory to medical treatment, but, if left untreated, it can lead to severe renal dysfunction and graft deterioration. The cause of transplant RAS appears to be multifactorial; suture technique, renal artery trauma during transplantation, kinking or twisting of the renal artery, rejection, atherosclerosis in donor or recipient arteries, and infection. Transplant RAS usually arises at the surgical anastomosis, although it can also occur in the donor renal artery and in the recipient iliac artery due to surgical trauma. In the case of a living donor, an end-to-end anastomosis with internal iliac artery is usually preferred, although there is no complete agreement between authors on the best surgical technique (3). An alternative arterial anastomosis can be performed between renal donor artery and recipient iliac external artery (end-to-side) (Fig. 1). Percutaneous transluminal angioplasty (PTA) represents the first-line treatment option; aim of the procedure is to recover renal function and control blood pressure. Technical success and complication rates are estimated between 60-94% and 0-8.3%, respectively (Fig. 2) (4). Restenosis develops from 10% to 12% of the patients treated with PTA, commonly within 9 months from the procedure; PTA with stent deployment should be considered in these cases (5). Some authors reported the use of cutting-balloon angioplasty in pressure-resistant stenosis, with good result at mid-term follow-up (6).
Arterial and venous kinks originate from vascular redundancy, or when a shift of the graft and/or pelvic contents with time produces torqueing of the artery or vein (7). It may not be easy to distinguish a kink from a simple stenosis (Fig. 3). Surgery still remains as the treatment of choice for vascular kinks. When surgery is not feasible or patients refuse it, interventional techniques may be attempted, even though PTA is usually ineffective with an increased risk of arterial vasospasm and/or dissection.
Pseudoaneurysms arise from the iliac vessels involved in the anastomosis with donor renal artery and are a rare complication after transplantation, occurring in < 1% of all cases (8). Treatment is needed in order to prevent rupture; endovascular treatment options include stent-graft placement, ultrasound (US)-guided thrombin injection and coil embolization (Fig. 4). Even if sporadic cases of graft salvage exist, most patients require nephrectomy in order to prevent pseudoaneurysm rupture (9).
Dissection of the external iliac artery is a rare vascular complication after renal transplantation, which may cause graft ischemia owing to the absence of collateral circulation and also may compromise blood flow to the lower limbs. However, it has only been sporadically reported in literature (10). Most frequent cause is traumatic handling during surgery (arteriography or arterial claps) and the main interventional treatment is represented by PTA, usually followed by stenting (Fig. 5).
Even with the recent discoveries in non-invasive diagnostic techniques, core needle biopsy still remains as the gold-standard for diagnosing renal transplant dysfunctions. Arteriovenous fistulas and pseudoaneurysms arise from 1% to 18% of all renal graft biopsies, and in up to 30% of cases, they may coexist (11, 12). These conditions can be asymptomatic, but they sometimes may also cause gross hematuria and hypovolemia due to shunting; or less frequently, they can produce renal insufficiency and hypertension. The best treatment option is represented by super-selective coils embolization with a coaxial catheter, thus avoiding loss of normal functioning graft tissue and preventing reflux of embolization material in non-targeted branches (Fig. 6) (12). Technical success rate is reported from 71% up to 100% of patients, with symptom relief in 57-88% of all cases (12).
Renal allograft vascular thrombosis represents an unusual but severe complication, with their incidences reported between 0.4% and 6%, occurring more frequently during the first ten days after transplantation (13). Vascular thrombosis usually becomes evident as a sudden oliguria or anuria with reduction of graft function. Venous graft thrombosis usually develops from a deep venous thrombosis, which is not uncommon in the post-transplant setting, causing obstruction of the renal allograft vein. Surgical venous thrombectomy or, in the cases of less clot burden, catheter-directed thrombolysis have been employed; in anyway, a successful salvage of the transplanted kidney still remains unusual (Fig. 7) (14). If graft arterial thrombosis is recognized early, it is usually treated with surgical thrombectomy in order to restore tissue perfusion; however, several reports suggest catheter-guided fibrinolytic treatment in acute arterial graft thrombosis, with successful restoration of function if performed within 24 hours (Fig. 8) (15).
Ureteral stenoses occur in 2-10% of all transplanted kidneys and are generally classified as early (≤ 3 months) and late (> 3 months), depending on time of onset after transplantation; they have relatively different causes and prognosis (16). In fact, early stenoses tend to be a result of mechanical causes, such as kinks, edema, blood clots, or restrictive submucosal tunnel. They are generally located at ureterovescical junction, whereas late stenoses are provoked by generalized or focal fibrosis, resulting from ischemia or rejection, and are often localized at a more proximal site. Percutaneous interventional techniques are considered as the first-line treatment options because of their high success rates of between 58% and 95%, with a low risk of further complications. Interventional management of ureteral stenosis consists of percutaneous nephrostomy, balloon dilatation, insertion of a double-J stent, and rarely, metallic stent placement (Fig. 9).
Ureteral leak represents an early complication of renal transplant and is usually caused by ischemia at the distal third of the ureter or by technical failure during the uretero-neocystostomy anastomosis (Fig. 10) (16, 17). Although leaks are usually treated with reconstructive surgery, percutaneous deployment of a 8-12-Fr nephrostomy/nephroureterostomy in the transplanted kidney may be used as a first-line treatment, in order to divert urine away from the leak site, thus helping the spontaneous closure of the ureteral defect (Figs. 11, 12). The catheter should be left in place until leak resolution, after which it can be removed and a 6-10-Fr double-J stent can be deployed (16, 17).
Several perigraft fluid collections can arise following renal transplantation, which includes lymphocele, hematoma, abscess, and urinoma. Perigraft fluid collections are very common in the post-transplantation period, with a reported incidence up to 50% of all renal transplants in some series; 15-20% of them are symptomatic (18). Symptoms may include pain, especially in the case of infections, or obstructive symptoms due to compression of other organs. Hematoma, abscess, and urinoma present early in the post-transplantation period, while lymphoceles generally occur later. During post-transplant period, US and CT are the imaging tools more frequently used to evaluate complications. These tools can easily detect perigraft fluid collections, which more often show non-specific aspects. The imagings usually show simple or complex cystic fluid-density/anechoic structures. Needle aspiration of fluid collections under US or CT guidance is essential for specific diagnosis. Treatment depends on the type of perigraft collection. Lymphoceles occur in 1% to 26% of all renal transplantations, and they are usually small and asymptomatic, discovered incidentally on routine post-transplantation imaging examinations. The cause is not clearly understood, but they may result from transection of lymphatic vessels adjacent to external iliac vessels during transplantation, with subsequent lymphatic leakage and accumulation in the extra-peritoneal space next to the transplanted kidney (19). Only the large, symptomatic lymphoceles require treatment, including needle aspiration under US or CT guidance, which are eventually completed with sclerotherapy or drainage catheter deployment (Fig. 13). Surgical treatment is more invasive, and it aims at creating a window between lymphocele and peritoneal cavity. In the case of infection, any perigraft fluid collection may turn into a retroperitoneal abscess. Clinical features may vary depending on the location, but fever, pain, leukocytosis, and increased erythrocyte sedimentation rate are usually present. Although the appearances of sterile and infected collections may be overlapping, some criteria Chave been highlighted (20). Typically, an abscess appears on US-imaging as an anechoic mass with irregular margins and more or less internal echoes. CT usually shows a fluid collection with internal density between 0-25 Hounsfield units and late-enhancing irregular peripheral rim. Gas may be present within the lesion. Adjacent fascial planes may be obliterated or thickened. Standard treatment consists of percutaneous drainage under US or CT guidance, combined with systemic antibiotic therapy (Fig. 14). Hematomas are usually present early in the post-transplantation period and are usually small and asymptomatic. Reported incidence of significant postoperative hematomas from renal transplant varies from 4% to 8% (18). Hematomas may show variable features on US imaging, but they usually appear as complex mass with multiple septations. Percutaneous drainage represents the standard treatment and is only needed in the case of large and symptomatic hematomas, given the relatively high risk of infection related to the procedure. Deployment of a 12-14-Fr percutaneous drain is usually satisfactory in most cases. Urinomas are produced by urine leaks; imaging features are usually nonspecific on CT and US, but urinomas only rarely contain any internal septa. The treatment has been already discussed above.
Renal transplantation is the best choice of treatment for Cend-stage renal disease. Even with the recent advances of surgical techniques and immunosuppressive therapies, complications occur in 12-20% of the patients, which are mainly represented by urological complication (4-8%) and vascular complication (1-2%), and they can seriously impair patient's survival. It is mandatory for operators to be familiar with indications and limitations of percutaneous interventional procedures, in order to evaluate potential technical problems and their adequate solutions, thereby decreasing early and late complication rates.
Figures and Tables
Fig. 1
Volume-rendered image of transplantation kidney shows end-to-side anastomosis between donor renal artery and recipient external iliac artery.
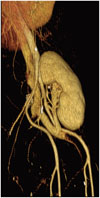
Fig. 2
67-year-old male, who received transplant 3 years before, is presented.
A, B. Maximum intensity projection and volume rendered CT images demonstrate severe renal artery end-to-side anastomotic stenosis (arrows). C-F. DSA images: ipsilateral approach is usually preferred to access end-to-side anastomosis with internal iliac artery (as in this patient), whereas contra-lateral approach is usually best for accessing end-to-side anastomosis with external iliac artery. DSA image confirms presence of severe stenosis at site of anastomosis (arrow in C). PTA procedure was performed with successful restoration of renal artery lumen (arrow in D) and final deployment of mono-rail pre-mounted balloon-expandable stent (5 × 18 mm; Tsunami, Terumo, BTG), with minimal residual stenosis. Stent should be used in patients with persistent systolic pressure gradient that exceeds 10 mm Hg after PTA, in case of flow-limiting dissection or residual stenosis > 30%. Pre-mounted balloon-expandable stents are preferred, which maximize deployment position and stability. DSA = digital subtraction angiography, PTA = percutaneous transluminal angioplasty
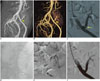
Fig. 3
50-year-old female is shown, 2 days after transplantation.
A, B. Duplex ultrasound images show tardus and parvus waveform in intra-parenchymal renal arteries, with increased velocity and turbulence in main transplant renal artery. C. Three-dimensional MIP reformatted image demonstrates kinking (arrow) of transplanted renal artery. MIP = maximum intensity projection
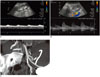
Fig. 4
33-year-old female with previous left renal graft explantation.
A, B. MIP and VR reformatted images illustrate large extrarenal pseudoaneurysm arising at site of previous end-to-end anastomosis, as confirmed by digital subtraction angiography images (arrows). C-E. Successful endovascular treatment was performed, by placing iliac covered stent-graft. MIP = maximum intensity projection, VR = volume-rendered
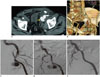
Fig. 5
47-year-old female is shown, at 1 day after transplantation with two renal arteries anastomosed to external iliac artery.
A, B. Three dimensional volume-rendered and maximum intensity projection reformatted images show post-surgery dissection (arrows) of left external iliac artery extending below 2 transplanted renal arteries, both originating from true lumen. C. CT findings were confirmed by digital subtraction angiography (arrow). D-H. Premounted stent (Express, Boston Scientific, USA) was deployed in proximal intimal flap, in order to allow expansion of true lumen and consequent collapsing and exclusion of false lumen.
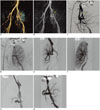
Fig. 6
40-year-old male is shown.
A. Selective angiogram of renal artery demonstrates large post-biopsy intra-renal pseudoaneurysm. B, C. After super-selective catheterization, embolization with 33% solution of N-butil cyanoacrylate glue (Glubran 2 + Lipiodol; 1:3, 1 mL) was performed, with complete deafferentation of pseudoaneurysm without any complications or non-target complications.
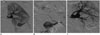
Fig. 7
48-year-old female is shown, at 2 days after transplantation.
A, B. Reformatted maximum intensity projection images show thrombosis of transplanted renal vein extending into external iliac vein (arrows), with markedly altered nephrogram of graft.
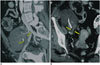
Fig. 8
70-year-old male is shown, who previously was surgically treated for large aortic aneurysm with aorto-iliac graft.
A-D. Multiplanar reconstruction and volume-rendered reformatted images show thrombosis of right iliac branch, involving transplanted renal artery (arrows in A, C), without any enhancement of renal graft (arrow in B), as confirmed by digital subtraction angiography (D). Endovascular thrombolytic therapy was unsuccessful; graft was explanted 1 week later.

Fig. 9
60-year-old male is shown, presenting with increased creatinine levels and decreased urine output, 9 months after transplantation.
A. Anterograde pyelogram shows distal ureteral stenosis with only minimal residual lumen. B, C. After accurate pre-dilatation of ureteral stenosis (percutaneous transluminal angioplasty-ballon: 5 × 40 mm, Wanda, Boston Scientific, USA) (B), double-J ureteral stent was successfully deployed (Flexima, Boston Scientific, USA) (C).

Fig. 11
49-year-old male is shown, who was complaining of pain and tenderness in right iliac fossa, 6 weeks after transplantation.
A, B. Unenhanced CT scans show large, heterogeneous, multiloculated fluid collection. After successful positioning of 12-Fr drainage catheter, aspiration material analysis demonstrated urine content, confirming diagnosis of urinoma. C. Procedure was completed by double-J urinary stent deployment (Flexima, Boston Scientific, USA) with resolution of leak.
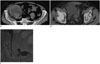
Fig. 12
65-year-old male is shown, at 25 days after transplantation.
A, B. Late-phase MIP reconstructions demonstrate contrast-material leakage from uretero-neocystostomy anastomosis into large pelvic fluid collection (arrows). Aspiration material from 8-Fr drainage tube confirms diagnosis of urinoma. C. After deployment of 8-Fr nephrostomy, anterograde pyelogram demonstrates presence of urinary leakage into pelvic fluid collection. Failure to catheterize bladder prevented deployment of urinary stent, so patient was surgically treated by ureteral reimplantation. MIP = maximum intensity projection

Fig. 13
51-year-old female is shown, at 43 days after transplantation.
A. Ultrasound (US) image shows large lymphocele, appearing as relatively anechoic multiloculated fluid collection compressing kidney. B. This complication was successfully treated by placing percutaneous drainage under US and fluoroscopic guidance.
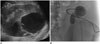
Fig. 14
51-year-old male is shown, at 23 days after transplantation, presenting with fever, neutrophil leukocytosis, and purulent discharge from surgical incision.
A. Ultrasound-image shows large perirenal fluid collection with hyperechoic content. B. 12-Fr double-lumen drainage cathether (vanSonnemberg, Boston Scientific, USA) was successfully deployed, with complete resolution of fluid collection in 4 days.
References
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999; 341:1725–1730.
2. Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, Shah H, et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001; 219:663–667.
3. Matheus WE, Reis LO, Ferreira U, Mazzali M, Denardi F, Leitao VA, et al. Kidney transplant anastomosis: internal or external iliac artery? Urol J. 2009; 6:260–266.
4. Rundback JH, Rizvi A, Tomasula J. Percutaneous treatment of transplant renal artery stenosis: techniques and results. Tech Vasc Interv Radiol. 1999; 2:91–97.
5. Salvadori M, Di Maria L, Rosati A, Larti A, Piperno R, Becherelli P, et al. Efficacy and safety of Palmaz stent implantation in the treatment of renal artery stenosis in renal transplantation. Transplant Proc. 2005; 37:1047–1048.
6. Peregrin JH, Bürgelová M. Restoration of failed renal graft function after successful angioplasty of pressure-resistant renal artery stenosis using a cutting balloon: a case report. Cardiovasc Intervent Radiol. 2009; 32:548–553.
7. Hedegard W, Saad WE, Davies MG. Management of vascular and nonvascular complications after renal transplantation. Tech Vasc Interv Radiol. 2009; 12:240–262.
8. Dimitroulis D, Bokos J, Zavos G, Nikiteas N, Karidis NP, Katsaronis P, et al. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009; 41:1609–1614.
9. Flechner SM, Novick AC. Renal transplantation. In : Gillenwater JY, Grayhack JT, Howards SS, Mitchell ME, editors. Adult and Pediatric Urology. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins;2002. p. 941–954.
10. Chen CH, Chen CH, Hsieh SR, Shu KH, Ho HC. Salvage of external iliac artery dissection immediately after renal transplant. Exp Clin Transplant. 2013; 11:274–277.
11. Grenier N, Claudon M, Trillaud H, Douws C, Levantal O. Noninvasive radiology of vascular complications in renal transplantation. Eur Radiol. 1997; 7:385–391.
12. Maleux G, Messiaen T, Stockx L, Vanrenterghem Y, Wilms G. Transcatheter embolization of biopsy-related vascular injuries in renal allografts. Long-term technical, clinical and biochemical results. Acta Radiol. 2003; 44:13–17.
13. Ojo AO, Hanson JA, Wolfe RA, Agodoa LY, Leavey SF, Leichtman A, et al. Dialysis modality and the risk of allograft thrombosis in adult renal transplant recipients. Kidney Int. 1999; 55:1952–1960.
14. Basić D, Hadzi-Djokić J, Milutinović D, Djokić M. [Vascular complications after kidney transplantation]. Srp Arh Celok Lek. 2003; 131:215–220.
15. Rouvière O, Berger P, Béziat C, Garnier JL, Lefrançois N, Martin X, et al. Acute thrombosis of renal transplant artery: graft salvage by means of intra-arterial fibrinolysis. Transplantation. 2002; 73:403–409.
16. Kaskarelis I, Koukoulaki M, Georgantas T, Bairamidis E, Kokkinos C, Ieronymou M, et al. Ureteral complications in renal transplant recipients successfully treated with interventional radiology. Transplant Proc. 2008; 40:3170–3172.
17. Bhagat VJ, Gordon RL, Osorio RW, LaBerge JM, Kerlan RK Jr, Melzer JS, et al. Ureteral obstructions and leaks after renal transplantation: outcome of percutaneous antegrade ureteral stent placement in 44 patients. Radiology. 1998; 209:159–167.
18. Yap R, Madrazo B, Oh HK, Dienst SG. Perirenal fluid collection after renal transplant. Am Surg. 1981; 47:287–290.
19. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000; 342:605–612.
20. Schwerk WB, Dürr HK. Ultrasound gray-scale pattern and guided aspiration puncture of abdominal abscesses. J Clin Ultrasound. 1981; 9:389–396.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download