Abstract
There is accumulating evidence that cancer stem cells (CSCs) play an integral role in the initiation of hepatocarcinogenesis and the maintaining of tumor growth. Liver CSCs derived from hepatic stem/progenitor cells have the potential to differentiate into either hepatocytes or cholangiocytes. Primary liver cancers originating from CSCs constitute a heterogeneous histopathologic spectrum, including hepatocellular carcinoma, combined hepatocellular-cholangiocarcinoma, and intrahepatic cholangiocarcinoma with various radiologic manifestations. In this article, we reviewed the recent concepts of CSCs in the development of primary liver cancers, focusing on their pathological and radiological findings. Awareness of the pathological concepts and imaging findings of primary liver cancers with features of CSCs is critical for accurate diagnosis, prediction of outcome, and appropriate treatment options for patients.
The concept of cancer stem cells (CSCs) has garnered a lot of attention in cancer research for the past decade in hopes that it may provide new insights into tumor biology which might lead to new anti-cancer treatment (1). CSCs are a subset of cancer cells within a cancer having the ability to self-renew and differentiate into a heterogeneous lineage of cancer cells that eventually form tumors (2). In addition to initiating tumor formation, CSCs have been shown to be responsible for maintaining tumor growth, generating distant metastases, and causing relapse after treatment (3). CSCs have been identified in primary liver cancers such as hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (CC). A cumulative body of evidence suggests that these liver cancers can originate from CSCs which themselves may be derived from hepatic stem/progenitor cells (HPCs) upon genetic deregulation of stem cell self-renewal pathway (4).
The most common primary liver cancers are HCC and CC defined as malignant neoplasms showing hepatocytic and cholangiocytic differentiation, respectively. Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is another distinct and rare primary liver cancer that shows a mixture of hepatocytic, cholangiocytic or intermediate (hepatocytic-cholangiocytic) phenotypes (5, 6). Recent studies using advanced stem cell biology-based technologies suggest that cHCC-CCs may arise from transformed HPCs responsible for the bi-phenotypic feature of these tumors (7, 8).
According to the current practice guidelines for the management of HCCs (9, 10), HCCs in cirrhotic livers can be diagnosed noninvasively through typical imaging features on dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) without histological confirmation. Until now, however, few studies have specifically evaluated the role of imaging or radiologic features of primary liver cancers of postulated CSC origin. A variety of imaging modalities including ultrasound (US), CT, MRI, and positron emission tomography (PET) are widely used for the detection, characterization, staging, and assessment of resectability of primary liver cancers including HCCs and CCs (11, 12, 13). Awareness of imaging characteristics of liver cancers with stem cell features is important for clinical practice. In addition, patients' prognoses differ among HCCs, CCs, and cHCC-CCs. Their features on cross sectional imaging would provide great value to the determination of the most appropriate treatment plan and the predication of the prognosis of each patient. Furthermore, prognosis after liver transplantation differs among patients with HCCs, CCs, and cHCC-CCs, and distinguishing among HCCs, CCs, and cHCC-CCs would be important for the selection of optimal candidates for liver transplantation in the organ allocation system (14, 15). Recent studies have reported that "stemness"-related markers expressing HCCs, CCs, and cHCC-CCs have different prognoses and responses to treatment compared to tumors that do not express these markers (16, 17, 18, 19). Therefore, identification of imaging biomarkers correlated with stem cell features is an area of great interest (20, 21).
In this review, we address the concept of CSCs in hepatocarcinogenesis which would help radiologists understand the current pathological classification of primary liver cancers, including tumors with histopathologic features intermediate between HCCs and CCs. In addition, as radiologic diagnosis is critical for the appropriate treatment and prediction of prognosis for primary liver cancer patients, the spectrum of imaging findings presented in primary liver cancers of postulated CSC origin will be discussed based on previously published studies.
Cancer stem cells are cancer cells that harbor properties of stem/progenitor cells, such as self-renewal, differentiation, proliferation, tumorigenicity, and chemoresistance. They have been identified in various hematologic and solid tumors, including primary liver cancers. The presence of CSCs has been associated with poor prognosis in cancer patients (16, 17, 18, 19). Different sources have been suggested for liver CSCs, such as HPCs, bone marrow-derived cells, and dedifferentiated hepatocytes. Liver CSCs are heterogeneous in cellular origins. They also demonstrate heterogeneity in marker expression (22, 23).
Hepatic stem/progenitor cells are hypothesized to reside in bile ductules and the canals of Hering. HPCs can differentiate into both hepatocytes and cholangiocytes. HPC component expanded in the setting of chronic liver disease can be seen microscopically in the form of ductular reactions (5). Repeated rounds of inflammation and regeneration in chronic liver disease result in various genetic and epigenetic changes to both parenchymal cells and HPCs, activating various signaling pathways due to the stromal microenvironment. These changes are thought to promote the development of liver CSCs (23). In addition, liver CSCs may be generated from non-CSCs through dedifferentiation (23).
The presence of CSCs is associated with aggressive biologic behavior, chemoresistance, and poor prognosis (16, 17, 18, 19). It is important to identify tumors with significant proportions of CSCs for both prognostic and therapeutic purposes. Increasing interest in stem cells has led to the identification of a large number of putative HPC markers, including epithelial cell adhesion molecules (EpCAM), CD133, CD90 (Thy-1), CD44, CD24, CD13 (aminopeptidase N), DLK-1, OV-6, keratin 19 (K19), K7, and others (22, 23, 24, 25, 26). Functional assays such as the isolation of side population cells by Hoechst dye staining are also currently used to select liver CSCs (27).
Extensive studies on HPC markers over the past decade have led to an increasing interest in the histological diversity of primary liver cancers. Now we have cumulative data on a wide spectrum of hepatic malignancies with various morphological and immunophenotypic features, a significant proportion of which seem to be related to HPCs (Fig. 1). These different primary liver cancers will be reviewed in the following in details.
Combined hepatocellular-cholangiocarcinoma, also known as mixed hepatobiliary carcinoma, is defined as a primary liver cancer consisting of unequivocal elements of both HCC and CC that are intimately admixed (28), with a reported incidence ranging from 1.0% to 6.5% in all primary liver cancers (29). cHCC-CC was first reported in the literature by Wells in 1903. Since then, it has been described under various names, including "combined liver cell and bile duct carcinoma" (6). Various classifications and definitions have been described for this tumor, possibly accounting for the vast heterogeneity in the incidence of cHCC-CCs reported thus far. For example, a classification by Allen and Lisa (6) included a separate HCC and CC in the same liver as a type of cHCC-CC. Fibrolamellar tumors (now a distinct variant of HCC; not further discussed in this review) were designated as "type III cHCC-CCs" in another classification (30).
The cellular origin of cHCC-CC is still unclear. However, several possible theories have been hypothesized: 1) HCC and CC components may arise from hepatocytes and cholangiocytes, respectively, and collide to form a single tumor; 2) HCCs may arise first and subsequently transform in part to CCs, or vice versa, and acquire features of HPCs (dedifferentiation theory); or 3) HPCs, capable of both hepatocellular and cholangiocytic differentiation, may undergo malignant transformation and differentiate into HCC and CC and components with HPC features (maturation arrest theory). Recent interest in HPCs and increasing evidence (morphological, immunohistochemical, and molecular) for HPC differentiation in cHCC-CCs has led to a wider acceptance of the maturation arrest hypothesis (5). In addition, increasing identification of HPC-like cells in cHCC-CCs has resulted in the designation of a new subtype of cHCC-CC in the latest World Health Organization (WHO) classification of tumors: "cHCC-CC with stem cell features" (28).
In the 2010 WHO classification (most recent version), cHCC-CC is largely classified into two types: 1) cHCC-CC, the classical type (Fig. 2); 2) cHCC-CC with stem cell features (Figs. 3, 4, 5). The classical type of cHCC-CC is characterized by the presence of typical HCC-like and typical CC-like areas within the same tumor (Fig. 2). Separate HCCs and CCs arising in the same liver are not included in this category. HCC and CC components are also immunohistochemically compatible with hepatocytic and cholangiocytic differentiation, respectively. For example, HCC components may express HepPar1 and alpha-fetoprotein (AFP) showing canalicular patterns on CD10 or polyclonal-carcinoembryonic antigen (CEA) immunostains, whereas CC components may express biliary markers such as K19, K7, and CEA. Interestingly, in the majority of these classical cHCC-CCs, careful microscopic evaluation often reveals transitional areas that are morphologically intermediate between HCCs and CCs. Tumor cells in these areas often have morphological features that are similar to HPCs (small cells with increased nuclear/cytoplasmic ratio and hyperchromatic nuclei) expressing immunohistochemical markers of progenitor cell differentiation, such as K19, neural cell adhesion molecules (CD56), EpCAM, and c-kit (31).
On the other hand, cHCC-CCs with stem cell features are predominantly composed of tumor cells with features of HPC differentiation. Three specific subtypes of cHCC-CC with stem cell features described in the current WHO classification are typical, intermediate-cell, and cholangiolocellular subtypes (28). Although it should be stressed that these subtypes are not currently considered to be distinct clinicopathologic entities, no biological difference among these three subtypes has been reported so far. It remains to be seen whether further subtyping of cHCC-CCs with stem cell features will actually have clinical implications. In addition, these subtypes may only represent a few of the heterogeneous group of cHCC-CCs. In fact, new variants have recently been identified in the literature, including the newly described "ductal plate malformation subtype of cHCC-CC with stem cell features" (32).
The first subtype is the "typical subtype" that has been previously reported as "hepatic stem cell malignancies" (28, 33). This subtype is characterized by nests of mature-looking hepatocytes (resembling a typical HCC) that are surrounded by small cells with a high nuclear/cytoplasmic ratio and hyperchromatic nuclei in the periphery (Fig. 3). Interestingly, these small cells share morphologic and immunohistochemical features with HPCs, thus recapitulating the appearance of ductular reactions around hepatocytes in chronic liver disease. Fibrous stromas surrounding the tumor cell nests are often abundant. The second subtype is the "intermediate cell subtype" which is composed of tumor cells with histological and immunohistochemical features intermediate between hepatocytes and cholangiocytes (Fig. 4) (31). These neoplastic cells are small, oval-shaped, and uniform with hyperchromatic nuclei and scanty cytoplasm. They are arranged in strands, trabeculae, or solid nests embedded in a desmoplastic stroma. These tumor cells express both hepatocytic and cholangiocytic markers on immunohistochemistry. They are also positive for markers associated with HPCs, suggesting a HPC origin. The third subtype is the "cholangiolocellular subtype" of cHCC-CC with stem cell features (Fig. 5) (28, 34, 35). This subtype is characterized by small cells morphologically similar to those described for the typical stem cell subtype. Cells are arranged in a distinct tubular or cord-like anastomosing architectural pattern recapitulating the canals of Hering or ductular reactions. These tubules or cords arranged in fibrous stromad have been reported to be immunohistochemically positive for HPC markers. Mucin production is absent in these tumors. HCC-like or CC-like areas are often present at the periphery of these tumors. These tumors have been previously classified as a special subtype of CC due to their tubular architecture with associated desmoplastic stroma. However, it is now regarded as a subtype of cHCC-CC with stem cell features. Increasing evidence suggests that they share morphological and immunohistochemical features of HPCs. They lack mucin production.
Combined hepatocellular-cholangiocarcinomas contain histological elements of both HCCs and CCs. Imaging findings of cHCC-CCs are also a coexistence or mixture of HCC and CC imaging features. Therefore, cHCC-CCs can mimic either HCCs or CCs on imaging (Fig. 6) (36). In addition, both HCCs and CCs can show a variety of radiologic features according to the tumor size, cellular differentiation, and underlying liver disease (37, 38, 39, 40), differential diagnosis between cHCC-CCs from HCCs or CCs through imaging is often difficult. cHCC-CCs have been frequently misdiagnosed as HCCs or CCs in preoperative settings. Fowler et al. (7) have reported that preoperative imaging diagnoses of cHCC-CCs showed 33-34% sensitivity with 81-100% specificity. In addition, Nishie et al. (41) reported a diagnostic accuracy of only 33% for cHCC-CCs through analysis of enhancement patterns within and around the tumor on contrast-enhanced CT.
Nevertheless, there is cumulative evidence of useful radiologic features that can assist in the differentiation of cHCC-CCs from HCCs and CCs. Considering the histopathological spectrum of primary liver cancers, cHCC-CCs would present a spectrum of imaging findings which are intermediate between HCCs and CCs (Figs. 3, 4, 5, 6) (7). The known imaging features of cHCC-CCs compared to HCCs and CCs are summarized in Table 1. Based on a previous study on CT findings, cHCC-CCs grossly resemble HCC, showing arterial enhancement and delayed washout in the entire portion of the tumor. However, cHCC-CCs grossly resemble CC, mostly showing arterial enhancement in the peripheral portion of the tumor and low attenuation or central enhancement on the delayed phase (42). On MRI, cHCC-CCs have moderately high signal intensity on T2-weighted images, peripheral or heterogeneous enhancement on arterial phase images, with tumor portions showing progressive contrast enhancement (43). Hwang et al. (44) reported that an irregular shape of the tumor with strong enhancement of the peripheral portion on the arterial phase in the absence of reverse target appearance (central enhancement with an hypo-intense rim) on hepatobiliary phase images of gadoxetic acid-enhanced MRI would suggest cHCC-CC rather than CC. Ancillary imaging findings are also helpful for diagnosis. Fukukura et al. (42) reported that the absence of intrahepatic bile duct dilation can be suggestive for cHCC-CC over intrahepatic CC. Absence of a pseudocapsule with a retraction of the hepatic surface and lymph node involvement can also raise suspicion for cHCC-CCs or CCs rather than HCCs (7, 43, 45). In addition, the presence of vascular invasion has been reported to be more frequent in the cHCC-CC group than in the HCC or CC groups (Fig. 7) (46, 47). Furthermore, 18F-fluorodeoxyglucose (FDG) PET has shown to be useful for cHCC-CCs which have higher FDG uptake than poorly differentiated HCCs (21). 18F-FDG PET is also useful for the detection of extrahepatic metastases in patients with cHCC-CCs (48).
Another subtype of interest is cholangiolocellular carcinoma (CLC), an extremely rare primary liver cancer. CLC was previously classified as a subtype of intrahepatic CC. The latest WHO classification has reclassified CLC as one of the stem-cell subtype of cHCC-CC (28). Although few characteristic imaging findings of each subtype of cHCC-CCs have been reported, imaging features of CLCs have been relatively more investigated than other subtypes of cHCC-CCs because CLCs resembles HCCs, particularly in patients with chronic liver disease or liver cirrhosis, or as a subtype of CCs showing atypical imaging features based on previous classification schemes (49, 50). Most CLCs are found in the peripheral portion of the liver which could be explained by the location of its cells of origin (HPCs are located in cholangioles or the canals of Hering) (20). On contrast-enhanced imaging or angiographic studies, considering that CLCs have histologically intermediate characteristics between HCCs and CCs, various dynamic enhancement patterns are expected depending on the pattern of tumor cell proliferation and the degree of fibrous stroma (50). Asayama et al. (50) reported that CLCs can show a variety of imaging features that resemble HCC and/or CC, i.e., they are either homogeneous, mosaic, or peripheral enhancement on arterial phase image of dynamic CT or MRI; they have delayed washout or concentric delayed filling; they have hypervascularity on CT hepatic angiography; they have portal perfusion defects on CT arterioportography. Motosugi et al. (49) have demonstrated that CLCs show HCC-like complete enhancement or CC-like peripheral enhancement on arterial phase and CC-like persistent enhancement on delayed phase of dynamic CT and MRI. Fukukura et al. (51) reported two cases of CLCs with obvious contrast enhancement in the peripheral portion of the tumor on the arterial and portal venous phases with concentric filling on the delayed phase. In summary, although imaging findings is not specific for the diagnosis of CLCs yet, a tumor located in the peripheral portion of the liver with HCC-like early enhancement and CC-like persistent delayed enhancement may suggest the possibility of CLCs (Figs. 5, 8). Sasaki et al. (52) recently reported that a proportion of subtypes with stem cell features of cHCC-CCs may affect clinicopathological factors. According to their study results, the CLC component in cHCC-CC was significantly correlated with the degree of fibrosis and inversely correlated with tumor size (52). Further studies on the correlation of histopathologic findings with corresponding imaging features of the different subtypes of cHCC-CCs are warranted.
Many efforts have been made to improve the preoperative differentiation performance of cHCCs from HCCs or CCs through a combination of radiologic findings with demographics and tumor markers, as current conventional imaging-alone has limitations of substantial overlaps in the diagnosis of cHCC-CCs from HCCs or CCs. Several previous studies have revealed that combined interpretation of imaging features and tumor markers including AFP and carbohydrate antigen (CA) 19-9 (frequently elevated in HCCs and CCs) could improve the diagnostic performance of cHCC-CCs (53, 54). For example, radiologic findings similar to HCC and/or CC together with simultaneous modest elevation of serum AFP and CA 19-9 have been suggested as reliable indicators of cHCC-CC.
Chronic viral hepatitis or underlying liver cirrhosis has been reported to be more common in patients with cHCC-CC and HCC than in those with CC (15, 54). Although demographic characteristics can provide important information for the diagnosis of liver tumors, chronic liver disease is a risk factor not only for cHCC-CC and HCC, but also for intrahepatic CC (55). Small intrahepatic CCs in the cirrhotic liver may show atypical imaging features mimicking those of HCC (56). Thus, imaging interpretation should be performed with caution for liver tumor if the patient has chronic liver disease.
In addition to microscopy which can readily used to identify cHCC-CCs, some morphologically typical HCCs may also show expressions of "stemness"-related markers, also known as HCCs with HPC immunophenotype (57) characterized by the expression of "stemness"-related markers, such as K19 (Fig. 9), CD133, and EpCAM. In > 5% of tumor cells, HCCs with these markers are associated with poorer prognosis compared to usual HCCs without these markers (58). HCCs with "stemness"-related markers are characterized by more frequent microvascular invasion, fibrous stroma, and less tumor pseudocapsule formation compared to conventional HCCs. They are known to behave aggressively, with higher stages at diagnosis and decreased overall survival rates (58). Associations between K19 expression and increased epithelial-mesenchymal transition-related proteins have been demonstrated in both Korean and Caucasian cohorts (58, 59). A recent Japanese study demonstrated that K19 expression in HCCs at liver biopsy was an indicator of high tumor recurrence rate after radiofrequency ablation (60). The frequency of K19 expression in HCCs varies from 4% (60) to 28% (58), depending on the cohort studied, the type of specimens used (resections, biopsies or tissue microarray cores), and the cut-off values determined for "positivity" (5% vs. 1%).
Identification of imaging features to differentiate HCCs with and without stem cell features would be clinically significant for the determination of patient management, prediction of tumor response to anti-cancer therapies, and prognosis stratification (22). Recently, Jeong et al. (61) reported differential MRI findings between HCCs with and without expression of stem cell markers. According to their study, HCCs with the expression of stem cell markers showed more frequent non-expanding gross morphology, persistent or progressive dynamic enhancement patterns, less frequent nodule-in-nodule appearance, and higher signal intensity on diffusion-weighted images than HCCs without the expression of stem cell markers (61). These features of HCCs with the expression of stem cell markers may be explained by their characteristic histologic features because the infiltrative growth pattern with less tumor pseudocapsule formation and abundant fibrous stroma are frequently found in HCCs with stem cell markers (Fig. 9) (58). Since the nodule-in-nodule appearance is considered to be a morphologic marker of the multistep process of hepatocarcinogenesis (62), the lower frequency of the nodule-in-nodule appearance in HCCs with stem cell markers may suggest that the main mode of HCC development with or without stem cell markers may be different. On gadoxetic acid-enhanced MRI, HCCs with the expression of stem cell markers were reported to have lower signal intensities on the hepatobiliary phase than those without the expression of stem cell markers (61). Choi et al. (63) demonstrated that gadoxetic acid-enhanced MRI could be useful to differentiate K19-positive from K19-negative HCCs because K19-positive tumors showed lower contrast enhancement ratios on hepatobiliary phase images than K19-negative tumors. This is somewhat consistent with the results of recent studies which have demonstrated that HCCs with lower signal intensities on hepatobiliary phase are associated with worse prognosis than those with higher signal intensities (64, 65). A meta-analysis also revealed that stem cell markers were associated with poor prognosis in patients with HCCs (66). Yamashita et al. (67) suggested that HCCs could be classified based on "stemness" status using a combination of the signal intensity on hepatobiliary phase images and serum levels of AFP. They revealed that HCCs with lower signal intensities on hepatobiliary phase and higher AFP were associated with the expression of "stemness"-related markers and poor prognosis (67). Imaging features of HCCs with "stemness"-related marker expression compared to those without "stemness"-related marker expression are summarized in Table 2.
Scirrhous HCC, a subtype of HCC with significant proportions of fibrous stroma within the tumor, has been reported to account for 4.6% of all HCCs (68). However, the definition of scirrhous HCC has varied in the literature. The minimum amount of intratumoral fibrous component required ranged from 30% to 50% in various definitions (38, 68, 69, 70, 71). Morphologically, in addition to marked stromal fibrosis, these tumors are often without encapsulation and atrophy of tumor cell trabeculae (Fig. 10). There is accumulating evidence that scirrhous HCCs also express HPC markers, leading to the suggestion that scirrhous HCCs may have stem-cell traits. Small HPC-like cells are also observed at the periphery of tumor cell nests (71, 72). Scirrhous HCCs have been linked to clinicopathological features with more aggressive behavior, such as more frequent vascular invasion, more infiltrative growth, and decreased disease-free survival compared to conventional HCCs (71).
Since there is a remarkable overlap in the morphological and immunophenotypic features among scirrhous HCCs, HCCs with "stemness"-related marker expression, and the typical stem cell subtype of cHCC-CC with stem cell features, it is possible that these tumors lie on the same spectrum of primary liver cancers, with conventional HCCs on the far left, followed by HCCs with "stemness"-related marker expression (little fibrous stroma), scirrhous HCCs (abundant fibrous stroma), cHCC-CCs, and CCs on the far right (Fig. 1) (71).
Common CT findings of scirrhous HCCs include ill-defined tumor margin, peripheral enhancement on arterial and portal phases, prolonged and delayed enhancement on the delayed phase, and hepatic surface retraction. However, the presence of a washout area or tumor pseudocapsule enhancement is rare, significantly differentiating it from typical HCCs (Fig. 10) (38). Therefore, scirrhous HCCs often mimic intrahepatic CCs as well as cHCC-CCs on imaging studies. According to Park et al. (73), although scirrhous HCCs may resemble CCs as both have lobulating shapes, rim enhancement, and target appearance on diffusion weighted images, but arterial hyperenhancement of the tumor diameter at 20% or greater may suggest scirrhous HCCs rather than CCs. The target appearance on hepatobiliary phase images defined as peripheral rim-like hypointensity compared to the central portion with contrast enhancement was also reported to be a potential imaging finding suggesting CCs rather than scirrhous HCCs according to a previous study using gadobenate dimeglumine-enhanced MRI by Jeon et al. (74). However, Park et al. (73) reported that the target appearance on hepatobiliary phase images may not be a useful finding to differentiate scirrhous HCCs from CCs as both tumors can show target appearance in approximately 80% of cases, reflecting the similar histologic findings between of the two tumors, i.e., abundant central fibrosis and rich tumor cellularity in the peripheral portion.
Intrahepatic CCs are at the other end of the "primary liver cancer spectrum". Recent evidence suggests that intrahepatic CCs are also heterogeneous, as is the biliary tree itself: mucin-producing cholangiocytes are in large bile ducts; non-mucin-producing cuboidal cholangiocytes are in the small interlobular bile ducts; ductules contain HPCs. A recent study demonstrated that intrahepatic cholangiocarcinoma have heterogeneous clinicopathological, immunohistochemical, and molecular profiles depending on their postulated cells of origin (20). While tumors composed entirely of mucin-producing adenocarcinomas have similar profiles as hilar CCs (originating from mucin-producing biliary epithelium), those characterized histologically by a mixture of components (mucin-producing adenocarcinomas, ductular differentiation and hepatocytic differentiation) in the same tumor were shown to have similar profiles as those of cholangiolocellular carcinoma (20), which is now a subtype of cHCC-CC with stem cell features based on the latest WHO classification.
Compared to cHCC-CCs or HCCs with the expression of stem cell markers, the concept of CSCs in the development of CCs has been less well established. Therefore, few studies have detailed the imaging features of CCs with stem cell features. Recently, Komuta et al. (20) demonstrated that a substantial proportion of intrahepatic CCs displayed histological diversity with a mixed features of hepatocytic differentiation and ductular areas. These mixed CCs predominantly located in the peripheral portion of the liver were larger than classic intrahepatic CCs (mucinous CCs). On MRI, different imaging characteristics between mucinous CCs and mixed CCs were observed (20, 75). Due to the mucin component within the tumors, mucinous CCs frequently showed homogeneously high signal intensity on T2-weighted images, whereas mixed intrahepatic CCs frequently had heterogeneous signal intensity. In addition, on dynamic MRI, mucinous CCs showed peripheral enhancement on the arterial phase and concentric fill-in on the delayed phase, whereas mixed CCs had either peripheral, diffuse or nodular arterial enhancement and washout on the delayed phase in various patterns (20, 75). These imaging features may lead to misdiagnosis of intrahepatic CCs as HCCs, especially in patients with chronic liver disease.
Intrahepatic CCs arising from the background of chronic hepatitis or liver cirrhosis have been reported to frequently express stem cell markers, which support the concept of CSCs from HPCs in the carcinogenesis of CCs (76, 77). In terms of imaging findings, previous studies have revealed that intrahepatic CCs in chronic liver disease can have different imaging characteristics compared to tumors developed in the normal liver which have a vascular pattern similar to HCCs on contrast-enhanced US (78). Kim et al. (40) reported that the incidence of chronic liver disease was higher in the atypical enhancement group of intrahepatic CCs (arterial enhancement on dynamic contrast-enhanced CT in > 50% of the lesion volume) than in the typical enhancement group. In addition, a previous study showed that the differential diagnosis of small intrahepatic CCs in the cirrhotic liver from HCCs was difficult solely through contrast-enhanced CT because there were no significant difference in enhancement patterns between the two disease entities (Fig. 11) (56). Therefore, further studies are warranted to reveal the precise relationship between the expression of stem cell markers and HCC-like enhancement patterns of intrahepatic CCs in cirrhotic liver diseases.
In the grey zone between typical HCCs and CCs, there are a variety of primary liver cancers with histopathologic and imaging features that are a mixture of, or intermediate between tumors of hepatocytic and cholangiocytic differentiation, such as cHCC-CCs, HCCs with "stemness"-related marker expression, and a subtype of CCs with mixed hepatocytic differentiation and ductular areas. Familiarity with the imaging characteristics of these tumors and understanding the concept of HPCs and CSCs would be of clinical importance for early and accurate diagnosis, appropriate treatment, prediction of prognosis, as well as development of new anti-cancer drugs. For cases without typical imaging features of HCCs or CCs, histologic confirmation is suggested.
Figures and Tables
 | Fig. 1Spectrum of primary liver cancers according to cellular differentiation. CC = cholangiocarcinoma, cHCC-CC = combined hepatocellular-cholangiocarcinoma, HCC = hepatocellular carcinoma, WHO = World Health Organization |
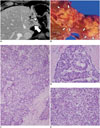 | Fig. 2Pathologically confirmed classical type of cHCC-CC.
A. Portal phase CT image showing ill-defined mass (arrow) in left lobe of liver and dilated intrahepatic duct (arrowhead). B. On gross specimen showing ill-defined infiltrative solid mass (arrows). C-E. Microscopic findings revealing mixture of glandular (CC) and hepatocytic (HCC) differentiation. CC component (D) and HCC component (E) are shown in greater detail on right panel (H&E stain, × 100 (C), × 200 (D, E)). CC = cholangiocarcinoma, cHCC-CC = combined hepatocellular-cholangiocarcinoma, HCC = hepatocellular carcinoma
|
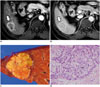 | Fig. 3cHCC-CC with stem cell features of typical stem cell subtype in 58-year-old male patient with chronic hepatitis B with moderate elevation of serum AFP level (154 IU/mL).
A. MR image on arterial phase showing 4 cm tumor (arrow) with peripheral enhancement in segment 6 of liver. B. Hepatic mass (arrow) showing centripetal enhancement on portal phase image. C. Photograph of gross specimen showing lobulated solid yellowish-white mass. D. On microscopic examination, tumor is composed of sheets and islands of polygonal mature-looking hepatocyte-like tumor cells (MH) with eosinophilic cytoplasm surrounded by peripheral rim of smaller tumor cells with high nuclear:cytoplasmic ratio resembling hepatic stem/progenitor cells (arrows) (H&E stain, × 400). AFP = alpha-fetoprotein, cHCC-CC = combined hepatocellular-cholangiocarcinoma
|
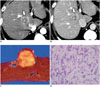 | Fig. 4Combined hepatocellular-cholangiocarcinoma with stem cell features of intermediate cell subtype in 53-year-old male patient.
A. Arterial phase CT scan demonstrating hyperenhancing mass (arrow) in segment 8 of liver. B. Portal phase CT scan showing prolonged enhancement of mass without washout (arrow). C. On gross examination, homogeneous yellowish-white solid lobulated mass is seen. D. Trabeculae and cords of relatively monomorphic population of tumor cells with high nuclear:cytoplasmic ratio in background of fibrotic stroma are seen (H&E stain, × 400).
|
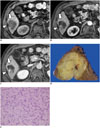 | Fig. 5Combined hepatocellular-cholangiocarcinoma with stem cell features of cholangiolocellular subtype in 67-year-old male patient with alcoholic liver cirrhosis.
A. Precontrast T1-weighted MRI showing lobulated well-defined mass (arrow) in segment 6 of liver. Note hepatic surface retraction (arrowhead). Arterial (B) and delayed phase images (C) demonstrating gradual enhancement of lateral portion of tumor (arrows). D. Solid greyish-white mass with indistinct margins. E. Tumor is characterized by distinct tubular structures embedded in fibrotic stroma, reminiscent of ductular reactions (H&E stain, × 200).
|
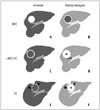 | Fig. 6Schematic representation of imaging features of HCC, cHCC-CC, and CC.
A, C, E. Arterial phase images. B, D, F. Portal and delayed phase images. A, B. HCC in cirrhotic liver shows arterial enhancement and delayed washout. Pseudocapsule shows delayed enhancement. C, D. cHCC-CC in cirrhotic liver shows strong arterial enhancement in peripheral portion of tumor and concentric filling on delayed phase. Note tumor thrombus in portal vein. E, F. CC in noncirrhotic liver shows weak arterial enhancement in peripheral portion of tumor and concentric filling on delayed phase. Note bile duct dilation and hepatic surface retraction. CC = cholangiocarcinoma, cHCC-CC = combined hepatocellular-cholangiocarcinoma, HCC = hepatocellular carcinoma
|
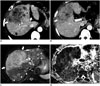 | Fig. 7Pathologically confirmed cHCC-CC with vascular invasion and lymph node metastases in 60-year-old male patient with liver cirrhosis.
A, B. Contrast-enhanced CT images of different sections showing large lobulated mass (arrows) with poor enhancement. A. Note tumor thrombus in portal vein branch in segment 8 (white arrowhead) and in middle hepatic vein (black arrowhead). B. Metastatic hepatoduodenal lymph node (arrowhead). C. Fat-suppressed T2-weighted image showing large high signal intensity mass involving segment 4 and right anterior segment of liver (arrows) as well as multiple daughter nodules (arrowheads). D. ADC map showing diffusion restriction of hepatic mass (arrow) and metastatic hepatoduodenal lymph node (arrowhead). ADC = apparent diffusion coefficient, cHCC-CC = combined hepatocellular-cholangiocarcinoma
|
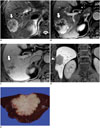 | Fig. 8Pathologically confirmed combined hepatocellular-cholangiocarcinoma with stem cell features of cholangiolocellular subtype in 58-year-old male patient with chronic hepatitis B.
A. Fat-suppressed T2-weighted image revealing lobulated mass (arrow) in right posterior segment of liver with moderately high signal intensity in peripheral portion of mass. On arterial phase (B) and portal phase (C) of gadoxetic acid-enhanced MR T1-weighted images, mass demonstrates strong arterial enhancement in peripheral portion and delayed concentric enhancement without washout (arrows). D. Hepatobiliary phase T1-weighted image showing mass as clear hypointense lesion. Note hepatic surface retraction (arrowhead). E. Solid lobulated on gross examination.
|
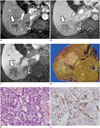 | Fig. 9Hepatocellular carcinoma with "stemness"-marker expression in 48-year-old male patient with liver cirrhosis.
A-C. Gadoxetic acid-enhanced MRI showing irregular mass (arrows) in segment 6 of liver with tumor thrombus (arrowheads) in right inferior hepatic vein. Mass shows peripheral enhancement on arterial phase (A) and delayed enhancement (B). C. Hepatobiliary phase showing mass with hypointensity. D. Gross examination reveals infiltrative solid greyish-white mass in liver. E. Tumor cells with eosinophilic cytoplasm arranged in sheets and pseudoglandular structures (H&E stain, × 400). F. Immunohistochemical staining revealing K19-positive cells in tumor (× 400).
|
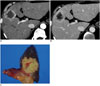 | Fig. 10Scirrhous hepatocellular carcinoma in 50-year-old female patient with liver cirrhosis.
A. Arterial phase CT image showing lobulated mass with peripheral enhancement in segment 8 of liver. B. Portal phase CT image demonstrating prolonged centripetal enhancement of tumor. C. Specimen photograph showing lobulated solid yellowish-white mass.
|
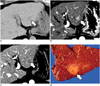 | Fig. 11Intrahepatic CC mimicking HCC in background of chronic liver disease in 63-year-old female patient.
A. Precontrast CT image showing small low-attenuated mass (arrow) in segment 2 of liver. Note surface nodularity of liver suggesting liver cirrhosis. B. Arterial phase CT image showing mass with hyperenhancement (arrow). C. Portal phase CT image demonstrating washout of mass (arrow). This dynamic enhancement pattern is suggestive of HCC. D. Specimen photograph revealing lobulated yellowish mass (arrow) which was pathologically confirmed as intrahepatic CC. Note background cirrhotic liver. CC = cholangiocarcinoma, HCC = hepatocellular carcinoma
|
Table 1
Comparison of Imaging Features of HCC, cHCC-CC, and Intrahepatic CC

Table 2
Comparison of Imaging Features of HCCs without and with Stemness-Related Marker Expression

Acknowledgments
We thank Yoon Jin Lee, M.D. for the preparation of the figures, and Chris Woo, B.A. for his editing.
References
1. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012; 12:133–143.
2. Hill RP, Perris R. "Destemming" cancer stem cells. J Natl Cancer Inst. 2007; 99:1435–1440.
3. Marquardt JU, Factor VM, Thorgeirsson SS. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol. 2010; 53:568–577.
4. Lo RC, Ng IO. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer. 2013; 2:84–92.
5. Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006; 25:3818–3822.
6. Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949; 25:647–655.
7. Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, et al. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013; 201:332–339.
8. Libbrecht L. Hepatic progenitor cells in human liver tumor development. World J Gastroenterol. 2006; 12:6261–6265.
9. Bota S, Piscaglia F, Marinelli S, Pecorelli A, Terzi E, Bolondi L. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer. 2012; 1:190–200.
10. Song DS, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012; 18:258–267.
11. Oliva MR, Saini S. Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging. 2004; 4(Spec No A):S42–S46.
12. Joo I, Choi BI. New paradigm for management of hepatocellular carcinoma by imaging. Liver Cancer. 2012; 1:94–109.
13. Joo I, Lee JM. Imaging bile duct tumors: pathologic concepts, classification, and early tumor detection. Abdom Imaging. 2013; 38:1334–1350.
14. Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003; 226:533–542.
15. Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006; 36:892–897.
16. Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006; 49:138–151.
17. Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, et al. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol. 2013; 140:329–340.
18. Fan L, He F, Liu H, Zhu J, Liu Y, Yin Z, et al. CD133: a potential indicator for differentiation and prognosis of human cholangiocarcinoma. BMC Cancer. 2011; 11:320.
19. Cardinale V, Carpino G, Reid L, Gaudio E, Alvaro D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol. 2012; 4:94–102.
20. Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012; 55:1876–1888.
21. Ijichi H, Shirabe K, Taketomi A, Yoshizumi T, Ikegami T, Mano Y, et al. Clinical usefulness of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for patients with primary liver cancer with special reference to rare histological types, hepatocellular carcinoma with sarcomatous change and combined hepatocellular and cholangiocarcinoma. Hepatol Res. 2013; 43:481–487.
22. Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012; 39:461–472.
23. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013; 123:1911–1918.
24. Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, et al. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2010; 51:1401–1409.
25. Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008; 2:333–344.
26. Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010; 59:953–962.
27. Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006; 44:240–251.
28. Theise ND, Nakashima O, Park YN, Nakanuma Y. Combined hepatocellular-cholangiocarcinoma. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC;2010. p. 225–227.
29. Lin G, Toh CH, Wu RC, Ko SF, Ng SH, Chou WC, et al. Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract. 2008; 62:1199–1205.
30. Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985; 55:124–135.
31. Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, et al. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004; 40:298–304.
32. Terada T. Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol. 2013; 6:737–748.
33. Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, et al. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003; 43:263–271.
34. Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008; 47:1544–1556.
35. Shiota K, Taguchi J, Nakashima O, Nakashima M, Kojiro M. Clinicopathologic study on cholangiolocellular carcinoma. Oncol Rep. 2001; 8:263–268.
36. Shetty AS, Fowler KJ, Brunt EM, Agarwal S, Narra VR, Menias CO. Combined hepatocellular-cholangiocarcinoma: what the radiologist needs to know about biphenotypic liver carcinoma. Abdom Imaging. 2014; 39:310–322.
37. Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH, Kim KW, et al. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012; 85:e573–e583.
38. Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009; 193:W482–W489.
39. Kim KE, Park MS, Bentley-Hibbert S, Baek SE, Kim YC, Kim MJ, et al. Hepatocellular carcinoma: clinical and radiological findings in patients with chronic B viral hepatitis and chronic C viral hepatitis. Abdom Imaging. 2012; 37:591–594.
40. Kim SA, Lee JM, Lee KB, Kim SH, Yoon SH, Han JK, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern--correlation with clinicopathologic findings. Radiology. 2011; 260:148–157.
41. Nishie A, Yoshimitsu K, Asayama Y, Irie H, Aibe H, Tajima T, et al. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. AJR Am J Roentgenol. 2005; 184:1157–1162.
42. Fukukura Y, Taguchi J, Nakashima O, Wada Y, Kojiro M. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr. 1997; 21:52–58.
43. de Campos RO, Semelka RC, Azevedo RM, Ramalho M, Heredia V, Armao DM, et al. Combined hepatocellular carcinoma-cholangiocarcinoma: report of MR appearance in eleven patients. J Magn Reson Imaging. 2012; 36:1139–1147.
44. Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, et al. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012; 36:881–889.
45. Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012; 19:2869–2876.
46. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005; 189:120–125.
47. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003; 33:283–287.
48. Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int. 2006; 26:781–788.
49. Motosugi U, Ichikawa T, Nakajima H, Araki T, Matsuda M, Suzuki T, et al. Cholangiolocellular carcinoma of the liver: imaging findings. J Comput Assist Tomogr. 2009; 33:682–688.
50. Asayama Y, Tajima T, Okamoto D, Nishie A, Ishigami K, Ushijima Y, et al. Imaging of cholangiolocellular carcinoma of the liver. Eur J Radiol. 2010; 75:e120–e125.
51. Fukukura Y, Hamanoue M, Fujiyoshi F, Sasaki M, Haruta K, Inoue H, et al. Cholangiolocellular carcinoma of the liver: CT and MR findings. J Comput Assist Tomogr. 2000; 24:809–812.
52. Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of 'subtypes with stem-cell feature' in combined hepatocellular-cholangiocarcinoma. Liver Int. 2014; 04. 08. [Epub]. http://dx.doi.org/10.1111/liv.12563.
53. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002; 94:2040–2046.
54. Tang D, Nagano H, Nakamura M, Wada H, Marubashi S, Miyamoto A, et al. Clinical and pathological features of Allen's type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg. 2006; 10:987–998.
55. Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005; 128:620–626.
56. Kim SJ, Lee JM, Han JK, Kim KH, Lee JY, Choi BI. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am J Roentgenol. 2007; 189:1428–1434.
57. Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis. 2010; 42:Suppl 3. S228–S234.
58. Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with "Stemness"-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011; 54:1707–1717.
59. Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014; 63:674–685.
60. Tsuchiya K, Komuta M, Yasui Y, Tamaki N, Hosokawa T, Ueda K, et al. Expression of keratin 19 is related to high recurrence of hepatocellular carcinoma after radiofrequency ablation. Oncology. 2011; 80:278–288.
61. Jeong HT, Kim MJ, Kim YE, Park YN, Choi GH, Choi JS. MRI features of hepatocellular carcinoma expressing progenitor cell markers. Liver Int. 2012; 32:430–440.
62. Kojiro M. 'Nodule-in-nodule' appearance in hepatocellular carcinoma: its significance as a morphologic marker of dedifferentiation. Intervirology. 2004; 47:179–183.
63. Choi JY, Kim MJ, Park YN, Lee JM, Yoo SK, Rha SY, et al. Gadoxetate disodium-enhanced hepatobiliary phase MRI of hepatocellular carcinoma: correlation with histological characteristics. AJR Am J Roentgenol. 2011; 197:399–405.
64. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology. 2012; 265:780–789.
65. Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, et al. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology. 2013; 267:776–786.
66. Ma YC, Yang JY, Yan LN. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2013; 25:1007–1016.
67. Yamashita T, Kitao A, Matsui O, Hayashi T, Nio K, Kondo M, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014; 60:1674–1685.
68. Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol. 2006; 21:1470–1477.
69. Matsuura S, Aishima S, Taguchi K, Asayama Y, Terashi T, Honda H, et al. 'Scirrhous' type hepatocellular carcinomas: a special reference to expression of cytokeratin 7 and hepatocyte paraffin 1. Histopathology. 2005; 47:382–390.
70. Okamura N, Yoshida M, Shibuya A, Sugiura H, Okayasu I, Ohbu M. Cellular and stromal characteristics in the scirrhous hepatocellular carcinoma: comparison with hepatocellular carcinomas and intrahepatic cholangiocarcinomas. Pathol Int. 2005; 55:724–731.
71. Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology. 2012; 55:1776–1786.
72. Fujii T, Zen Y, Harada K, Niwa H, Masuda S, Kaizaki Y, et al. Participation of liver cancer stem/progenitor cells in tumorigenesis of scirrhous hepatocellular carcinoma--human and cell culture study. Hum Pathol. 2008; 39:1185–1196.
73. Park MJ, Kim YK, Park HJ, Hwang J, Lee WJ. Scirrhous hepatocellular carcinoma on gadoxetic acid-enhanced magnetic resonance imaging and diffusion-weighted imaging: emphasis on the differentiation of intrahepatic cholangiocarcinoma. J Comput Assist Tomogr. 2013; 37:872–881.
74. Jeon TY, Kim SH, Lee WJ, Lim HK. The value of gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging for the differentiation of scirrhous hepatocellular carcinoma and cholangiocarcinoma with or without hepatocellular carcinoma. Abdom Imaging. 2010; 35:337–345.
75. Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology. 2012; 264:751–760.
76. Nomoto K, Tsuneyama K, Cheng C, Takahashi H, Hori R, Murai Y, et al. Intrahepatic cholangiocarcinoma arising in cirrhotic liver frequently expressed p63-positive basal/stem-cell phenotype. Pathol Res Pract. 2006; 202:71–76.
77. Xu J, Sasaki M, Harada K, Sato Y, Ikeda H, Kim JH, et al. Intrahepatic cholangiocarcinoma arising in chronic advanced liver disease and the cholangiocarcinomatous component of hepatocellular cholangiocarcinoma share common phenotypes and cholangiocarcinogenesis. Histopathology. 2011; 59:1090–1099.
78. Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010; 51:2020–2029.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download