Abstract
The radiologic findings of a single nodule from Pneumocystis jirovecii pneumonia (PJP) have been rarely reported. We described a case of granulomatous PJP manifesting as a solitary pulmonary nodule with a halo sign in a 69-year-old woman with diffuse large B cell lymphoma during chemotherapy. The radiologic appearance of the patient suggested an infectious lesion such as angioinvasive pulmonary aspergillosis or lymphoma involvement of the lung; however, clinical manifestations were not compatible with the diseases. The nodule was confirmed as granulomatous PJP by video-assisted thoracoscopic surgery biopsy.
Pneumocystis jirovecii pneumonia (PJP) is one of the most life-threatening infections in immunocompromised patients. Typical computed tomography (CT) findings of PJP include diffuse ground glass opacity in both lungs, but cystic formation or pneumothorax can also be seen, and nodules are found on rare occasion (1 ,2 ,3). Nodular opacities in Pneumocystis jirovecii (P. jirovecii) infection are related with granulomatous response to PJP infection on histopathologic examination that is an uncommon form of Pneumocystis infection. Solitary pulmonary nodule in patients with PJP infection is very rare and only 3 cases have been reported in the English literature (4, 5, 6). In addition, imaging findings of single nodular opacities in lymphoma patients can mimic lymphoma involvement of the lung or other infectious lesions. The diagnosis of granulomatous PJP infection is only possible by biopsy rather than traditional procedures like bronchoalveolar lavage (BAL), because the organisms are not infiltrated in the alveolar lumen (7).
We reported a patient with diffuse large B cell lymphoma (DLBL) who presented with an unusual manifestation of PJP infection after chemotherapy to stress the diagnostic importance of biopsy rather than BAL. The case was approved by the Institutional Review Board of Kyung Hee University Hospital that waived the requirement of patient informed consent for the retrospective investigation.
A 69-year-old woman was diagnosed with stage II DLBL involving the left gastric and paraaortic lymph nodes. She had received treatment with the R-CHOP regimen that consists of rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), adriamycin (50 mg/m2), vincristine (2 mg on day 1), and prednisolone (100 mg/day for 5 days) every 3 weeks. After her sixth cycle of R-CHOP chemotherapy, she was admitted to the hospital for management of neutropenia. Her absolute neutrophil count (ANC) was 81, but she had no fever (36℃) or any other symptoms. The C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) levels were in the normal range. There was no active lung lesion on chest radiograph. She was discharged after recovery of neutropenia (ANC 1771).
She underwent a routine chest CT 2 weeks later for evaluation of response to chemotherapy. There was a 1.3-cm subpleural solitary pulmonary nodule in the right lower lobe (Fig. 1A) on chest CT. Her body temperature was normal (36.2℃) and she was asymptomatic. Physical examination including lung sounds was normal. Laboratory examination revealed a hemoglobin level of 10.7 g/dL, a white blood cell count of 3240/µL (57.1% neutrophils, 20.7% lymphocytes, 10.4% monocytes, 6.2% eosinophils, 1.3% basophils) and a platelet count of 184000/µL. The CRP and ESR levels were normal.
The patient underwent positron emission tomography-computed tomography for further evaluation of the nodule, and the nodule showed high uptake (standardized uptake values maximum 5.4) (Fig. 1B). Short-term follow-up with chest CT was conducted under suspicion of an inflammatory lesion or lymphoma involvement of the lung. The subpleural nodule grew rapidly to 2.5 cm after 10 days with peripheral ground glass opacity showing a halo sign (Fig. 1C). There were no additional abnormal findings in other parts of the lungs. Interval growth during the short term period was suggestive of an infectious lesion and a nodule with a halo sign suggested the possibility of fungal infection such as angioinvasive pulmonary aspergillosis. However, neutrophil count was in the normal range and the aspergillosis antigen test was negative.
Finally, video-assisted thoracoscopic surgery biopsy and pathology results showed foamy eosinophilic exudates with the granuloma (Fig. 1D) and demonstrated P. jirovecii organisms on Gomori's methenamine silver nitrate stain (Fig. 1E). The diagnosis was granulomatous PJP infection. She received trimethoprim-sulfamethoxazol for 2 weeks and no additional infections developed during the follow-up period.
The most common manifestation of PJP is diffuse alveolar pneumonia with classical pathologic findings of foamy intra-alveolar eosinophilic exudates that contains PJP organisms (8, 9). Granulomatous reaction to PJP organisms is an unusual pathologic finding comprising 3-5% cases, predominantly in human immunodeficiency virus (HIV) patients (10). Until now, granulomatous PJP infection was reported less frequently in non-HIV than in HIV patients.
The pathogenesis of granulomatous reaction in PJP infection is related to host states such as prophylaxis with pentamidine, active malignancy, recent corticosteroid use, or immune reconstitution diseases rather than PJP genotypes (9). The systemic use of corticosteroids is associated with granulomatous PJP infection. The study patient was also on prednisolone for lymphoma treatment. Withdrawal from steroids can affect CD4 count and function and can cause immune reconstitution-like syndrome that is a phenomenon similar to immune reconstitution syndrome in HIV patients on anti-retroviral therapy (11). Insidious presentation with minimal symptoms of mild cough and dyspnea is common in granulomatous PJP infection; however, we experienced a very rare asymptomatic PJP infection with granulomatous reaction, the second case reported to date (12).
There are few reports on detailed imaging findings of granulomatous PJP infection, but granulomatous PJP infection shows variable radiological findings of mostly diffuse infiltrations, multiple nodules, and rarely a solitary pulmonary nodule (5, 13). This was the first report of granulomatous PJP presenting as a solitary pulmonary nodule with peripheral ground glass opacity, also called the halo sign. The halo sign on chest CT was first described in angioinvasive pulmonary aspergillosis, indicative of hemorrhagic infarction (14, 15). In addition, although it is less common, the CT halo sign may be observed in hemorrhagic nodules of infectious origin (mucormycosis, candidiasis, tuberculosis, viral pneumonia); hemorrhagic nodules of non-infectious origin (Wegener granulomatosis, Kaposi sarcoma, hemorrhagic metastases); tumor cell infiltration (lymphoma, adenocarcinoma with lepidic growth pattern); and non-hemorrhagic lesions (sarcoidosis and organizing pneumonia) (16, 17). The CT halo sign was associated with inflammatory cell infiltrates on histopathologic examination, in our case.
In case of granulomatous PJP infection, unlike PJP pneumonia that shows alveolar infiltration, organisms rarely exist in the alveoli lumen and therefore BAL specimens show high false negative rates (7). Furthermore, biopsy is needed for a definite diagnosis since PJP radiologically needs differentiation from other infections and malignancies like lymphoma. Physician awareness of atypical radiologic manifestation of granulomatous PJP infection will help in the diagnostic approach.
In conclusion, granulomatous PJP infection shows rare clinical and radiologic manifestation. Our experience suggests that granulomatous PJP infection should be considered a possible diagnosis when non-HIV immunocompromised patients present with a solitary pulmonary nodule with halo sign and when suspected, biopsy, rather than BAL should be performed promptly to prevent delay in diagnosis and effective treatment.
Figures and Tables
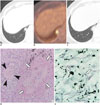
Fig. 1
69-year-old woman with granulomatous PJP.
Chest CT shows small subpleural nodule in right lower lobe (A) and shows high uptake on PET-CT (B). Follow-up CT taken 10 days later shows progression of nodule with peripheral ground glass opacity (C). Histopathologic features of granulomatous PJP show chronic granulomatous inflammation (white arrows) with foamy exudates (black arrowheads) (D, hematoxylin and eosin stain, × 400) and Pneumocystis cysts (white arrows) (E, Gomori methenamine silver stain, × 400). CT = computed tomography, PET-CT = positron emission tomography-computed tomography, PJP = Pneumocystis jirovecii pneumonia
References
1. Boiselle PM, Crans CA Jr, Kaplan MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999; 172:1301–1309.
2. Marchiori E, Müller NL, Soares Souza A Jr, Escuissato DL, Gasparetto EL, Franquet T. Pulmonary disease in patients with AIDS: high-resolution CT and pathologic findings. AJR Am J Roentgenol. 2005; 184:757–764.
3. Pastores SM, Garay SM, Naidich DP, Rom WN. Review: pneumothorax in patients with AIDS-related Pneumocystis carinii pneumonia. Am J Med Sci. 1996; 312:229–234.
4. Hartz JW, Geisinger KR, Scharyj M, Muss HB. Granulomatous pneumocystosis presenting as a solitary pulmonary nodule. Arch Pathol Lab Med. 1985; 109:466–469.
5. Chang H, Shih LY, Wang CW, Chuang WY, Chen CC. Granulomatous Pneumocystis jiroveci pneumonia in a patient with diffuse large B-cell lymphoma: case report and review of the literature. Acta Haematol. 2010; 123:30–33.
6. Bier S, Halton K, Krivisky B, Leonidas J. Pneumocystis carinii pneumonia presenting as a single pulmonary nodule. Pediatr Radiol. 1986; 16:59–60.
7. Hartel PH, Shilo K, Klassen-Fischer M, Neafie RC, Ozbudak IH, Galvin JR, et al. Granulomatous reaction to pneumocystis jirovecii: clinicopathologic review of 20 cases. Am J Surg Pathol. 2010; 34:730–734.
8. Bondoc AY, White DA. Granulomatous Pneumocystis carinii pneumonia in patients with malignancy. Thorax. 2002; 57:435–437.
9. Totet A, Duwat H, Daste G, Berry A, Escamilla R, Nevez G. Pneumocystis jirovecii genotypes and granulomatous pneumocystosis. Med Mal Infect. 2006; 36:229–231.
10. Travis WD, Pittaluga S, Lipschik GY, Ognibene FP, Suffredini AF, Masur H, et al. Atypical pathologic manifestations of Pneumocystis carinii pneumonia in the acquired immune deficiency syndrome. Review of 123 lung biopsies from 76 patients with emphasis on cysts, vascular invasion, vasculitis, and granulomas. Am J Surg Pathol. 1990; 14:615–625.
11. Otahbachi M, Nugent K, Buscemi D. Granulomatous Pneumocystis jiroveci Pneumonia in a patient with chronic lymphocytic leukemia: a literature review and hypothesis on pathogenesis. Am J Med Sci. 2007; 333:131–135.
12. Kumar N, Bazari F, Rhodes A, Chua F, Tinwell B. Chronic Pneumocystis jiroveci presenting as asymptomatic granulomatous pulmonary nodules in lymphoma. J Infect. 2011; 62:484–486.
13. Goodman PC. Pneumocystis carinii pneumonia. J Thorac Imaging. 1991; 6:16–21.
14. Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985; 157:611–614.
15. Primack SL, Hartman TE, Lee KS, Müller NL. Pulmonary nodules and the CT halo sign. Radiology. 1994; 190:513–515.
16. Parrón M, Torres I, Pardo M, Morales C, Navarro M, Martínez-Schmizcraft M. [The halo sign in computed tomography images: differential diagnosis and correlation with pathology findings]. Arch Bronconeumol. 2008; 44:386–392.
17. Pinto PS. The CT Halo Sign. Radiology. 2004; 230:109–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download