Abstract
Thyroid imaging is indicated to evaluate congenital hypothyroidism during newborn screening or in cases of a palpable thyroid mass in children and adolescents. This pictorial essay reviews the ultrasonography (US) of thyroid diseases in children and adolescents, including normal thyroid gland development, imaging features of congenital thyroid disorders (dysgenesis, [aplasia, ectopy, hypoplasia], dyshormonogenesis, transient hypothyroidism, thyroglossal duct cyst), diffuse thyroid disease (Grave's disease, Hashimoto's thyroiditis, and suppurative thyroiditis), and thyroid nodules. The primary imaging modalities for evaluating thyroid diseases are US and radionuclide scintigraphy. Additionally, US can be used to guide aspiration of detected nodules.
Imaging plays an important role in the evaluation of thyroid diseases in pediatric patients. Scintigraphy and ultrasonography (US) are the primary modalities used for thyroid screening and evaluating congenital hypothyroidism (CH) (1, 2, 3, 4). US and scintigraphy are complementary, as US provides anatomical information and scintigraphy provides functional information. In pediatric patients, US is a first-line diagnostic test for detecting thyroid abnormalities and lymphadenopathy. In addition, US can be used to guide aspiration of detected nodules.
The thyroid gland develops from the median and paired lateral anlages. Thyroid follicular cells originate as the median thyroid anlage, an endodermal thickening between the first and second pharyngeal arches during the fourth to fifth gestational week. A thickening rapidly forms a small out-pouch referred to as thyroid primordium (5, 6). This structure elongates into a bilobate diverticulum and descends caudally while maintaining contact with the aortic primordium. A small channel; i.e., the thyroglossal duct, temporarily manifests as a connection between the tongue and the caudal migration of the thyroid primordium, which then involutes. The thyroid primordium first courses anteriorly to the primordial hyoid bone and laryngeal cartilage, and then loops inferiorly and posteriorly to the hyoid bone before continuing its descent into the infrahyoid portion of the neck (5, 6). By the seventh week, the gland attains its normal final position anterior to the second and third tracheal rings (Fig. 1). Arrest of descent can occur anywhere from the tongue down to the lower neck. The thyroglossal duct has usually degenerated and disappeared at the time of descent by week 7. The proximal opening of the thyroglossal duct persists as a small pit in the tongue (i.e., the foramen cecum). Parafollicular cells originate as lateral thyroid anlages, also known as ultimobranchial bodies, which arise laterally at the fourth and fifth pharyngeal pouches around the gestational week 5. The lateral anlages merge with products of the median anlage after descent into the infrahyoid portion of the neck, resulting in parafollicular cells interspersed throughout the thyroid gland (5, 6).
Congenital hypothyroidism is a relatively common endocrine disorder that occurs in about 1 in 3981 live births in Korea (7). Neonatal screening started in 1985 in Korea. Most CH cases (75-80%) are caused by developmental defects known as thyroid dysgenesis. Dyshormonogenesis, which results from defective thyroxine (T4) synthesis, is caused by various autosomal recessive mutations (15-20%) (1, 2, 7). Thyroid dysgenesis is typically sporadic with a female predominance (1, 2) and there appears to be a wide range of molecular heterogeneity (1, 2, 3, 4). Among them, the thyroid-stimulating hormone receptor (TSHR) and thyroid peroxidase (TPO) genes account for the majority of mutation-positive cases of dysgenesis and dyshormonogenesis, respectively (8, 9). Scintigraphy reveals TSHR mutations in patients with decreased 99mTC-pertechnetate uptake and TPO mutations in those with increased uptake. TPO mutations are observed exclusively in patients with normal to enlarged thyroid glands. These organizational defects cause goitrous CH. Hypothyroidism resulting from dyshormonogenesis may cause neoplastic transformation if TSH levels are high for a prolonged period as a result of inappropriate levothyroxine treatment (4).
Ultrasonography and scintigraphy can differentiate various types of CH. Aplasia is defined as the failure to detect thyroid tissue on US and scintigraphy (Fig. 2). If the thyroid gland is not visible in the normal position, an attempt is made to find the ectopic thyroid by examining the thyroid migration pathway along the course of the thyroglossal duct (1, 2). Ectopic thyroid tissue can be found anywhere along the migration course of the thyroid primordium (Fig. 3); however, in most (90%) cases, it is located at the base of the tongue (6). The lingual thyroid is the only functioning thyroid tissue in approximately 75% of these patients. Appearance varies on US, but the tissue is generally located close to the hyoid bone. An ectopic gland appears as a round or oval area of uptake in the midline of the upper neck on scintigraphy (Fig. 3B). Scintigraphy is more sensitive than US for detecting an ectopic thyroid. The thyroid gland appears normal on US in patients with hypoplasia; however, scintigraphy reveals decreased isotope uptake. Transient CH is diagnosed by normal thyroid function, thyrotropin-releasing hormone studies, and scintigraphy performed during trial-off therapy. Trial-off therapy is conducted at 1-3 years for all children with suspected CH, except those with aplasia, to determine whether treatment remains necessary (Fig. 4). The percentage of transient CH cases is estimated to be 13-38% in the US (10, 11, 12). Thyroid hemiaplasia is generally discovered incidentally (Fig. 5), and thyroid function in children with thyroid hemiaplasia may decrease during puberty when the need for thyroid hormones is higher. Dyshormonogenesis involves an inborn error in thyroxine synthesis (1, 2). The most common defect is TPO deficiency, which results in the failure to oxidize iodide to iodine. Iodide becomes trapped in the thyroid gland and cannot be organified. US reveals an enlarged, orthotropic thyroid as a result of increased TSH level, and the lobes develop a convex appearance laterally (1, 2, 3, 4). The isthmus is readily visualized in contrast to a normal gland. Scintigraphy reveals an enlarged gland in the normal location with increased isotope uptake (Fig. 6). Thyroid gland size in a child varies with age and is correlated with height, weight, body surface area, and age (13, 14, 15, 16, 17). Enlargement of the thyroid gland can be visually assessed by anterior convexity instead of normal concavity, increased thickness of the isthmus, or tracheal narrowing.
The most common pitfall of isotope scanning is lack of uptake despite the presence of thyroid tissue, leading to the spurious diagnosis of aplasia. This phenomenon may occur for several reasons: when the scan is delayed beyond 4-5 days of T4 treatment; thus, TSH suppression has occurred by the time the scan is performed; from iodine exposure; from blocking TSHR antibodies causing transient hypothyroidism; and rarely, from defects affecting iodide uptake; e.g., sodium-iodide symporter gene mutations. The diagnosis of spurious thyroid absence will not be made if concurrent US is carried out (1, 2). Chang et al. (3) reported that 14% of pediatric patients with CH who had no uptake during scintigraphy had a thyroid gland in the appropriate location on US. Detecting the thyroid gland in the normal position provides greater diagnostic power to identify the etiology, decide when treatment should be discontinued, and determine the prognosis.
If the thyroglossal duct does not involute completely, the remnant may manifest as a thyroglossal duct cyst (TGDC), which accounts for approximately 70% of congenital abnormalities in the neck (6, 18). Eighty-five percent of TGDCs are located below the level of the hyoid bone and typically present as midline cystic masses. Anechoic or hypoechoic masses appear as well-circumscribed cysts deep or embedded in the strap muscle on US scans (Fig. 7). Acute infection, chronic inflammation, or hemorrhage may also occur. A ruptured cyst may result in a thyroglossal duct sinus that opens through the overlying skin. A localized mass appearing in a portion of the cyst wall suggests the possibility of a malignancy, whereas invasion of the
adjacent soft tissue with no history of infection strongly suggests the presence of a neoplasm in the cyst (18).
Detecting thyroid nodules has become more frequent with the widespread use of US. US is the mainstay for detecting and making the differential diagnosis of thyroid nodules and for providing biopsy guidance.
The incidence of thyroid nodules in children and adolescents is estimated to be between 1% and 18% (19, 20). Avula et al. (20) reported that a 1-year increase in age in children increases the risk of incidental thyroid abnormalities by 9%. The risk of malignant thyroid nodules in children ranges from 14% to 40%, which is considerably higher than that of adults (9-15%) (20, 21).
Ultrasonography findings for thyroid nodules are classified into one of three categories: probably benign, indeterminate, or suspicious malignant. Solid thyroid nodules with one or more US malignancy features (i.e., spiculated or microlobulated margin, markedly hypoechogenic, taller-than-wide shape, or calcification) are considered malignant, and thyroid nodules with one or two benign US features (i.e., complete cyst, predominantly a cystic lesion, cystic lesion with a comet-tail artifact, or spongiform benign cystic lesion) are considered probably benign (Fig. 8), based on the "Guidelines for Thyroid US" provided by the Korean Society of Thyroid Radiology (22). A nodule that does not show benign or malignant features is considered to be an indeterminate nodule. US findings are summarized in Table 1.
The majority (67.3%) of incidentally detected thyroid lesions in children are cysts (20). True epithelial-lined thyroid cysts are rare. Most cystic thyroid lesions are hyperplastic nodules that have undergone extensive degeneration. Colloid cysts may contain bright echogenic foci with comet-tail artifacts caused by the presence of microcrystals (Fig. 9) (23, 24).
An intrathyroid thymus has been reported in 17.3% of incidentally detected thyroid nodules (20). The thymus originates from the third pair of branchial pouches with a rudimentary portion arising from the fourth pair and descends to the superior mediastinum. Migration anomalies can produce an ectopic thymus that rests along the path of descent (18, 25). These lesions have US findings similar to a normal thymus and are more common in males than females, in contrast to the higher incidence of incidental thyroid lesions in adult females (Fig. 10).
Malignancies include papillary, follicular, and medullary carcinomas, and a papillary carcinoma is the most common (80%). Of thyroid cancer cases, 5-10% are familial and autosomal dominant; 17% are follicular, and 2-3% are medullary and often diagnosed in patients with multiple endocrine neoplasia syndrome (23).
Thyroid cancer is the eleventh most frequently diagnosed cancer in Korean children < 14 years of age and the fifth most common cancer in girls (26). The prevalence of thyroid cancer in girls increased from the tenth to the fifth most common cancer between 2002 and 2011. Thyroid cancer in children tends to present at a more advanced stage than that in adults and with a higher frequency of lymph node and pulmonary metastases. Distant metastasis is less common than regional lymph node involvement, and the lung is the most common site of distant metastasis. Pediatric patients with papillary thyroid cancer (PTC) have a good prognosis, despite a high incidence of cervical lymph node metastasis (27, 28). Metastatic nodes are characterized by a round shape, asymmetrical cortical thickening, hyperechogenecity, multiple punctate calcifications, cystic changes, loss of fatty hilum, or increased vascularity on color Doppler imaging (29). The reasons for the variations between children and adults are unknown, but are generally thought to be due to differences in mutations and oncogene expression in the thyroid (30, 31).
In addition to suspicious malignant nodules, a diffusely enlarged thyroid with numerous microcalcifications on US is defined as a thyroid malignancy and should be evaluated using fine-needle aspiration (FNA) biopsy with a BRAF analysis (31).
Papillary thyroid cancer is frequently associated with genetic alterations. The BRAF mutation is intrinsically associated with increased progression and aggressiveness of PTC. Similarly, the BRAF mutation has a high predictive value for PTC recurrence (32). The frequency of the BRAF mutation increases with age. The prevalence of the BRAF mutation in Korean children (30-50%) is lower than that in adults (70-83%) (31, 33).
Follicular carcinoma accounts for approximately 17% of thyroid cancers and is more common in females than males (23, 26, 27, 28). The distinction between a benign (Fig. 11) and malignant follicular neoplasm can only be made by evaluating the presence of capsular or vascular invasion during a histological examination. The estimated rate of malignancy for follicular neoplasm is variable, ranging from 10-30% (34, 35). Follicular thyroid tumors present with several genetic alterations, such as aneuploidy, RAS mutations, and PAX8/PPARγ rearrangements. Activation of RAS gene mutations plays an important role initiating follicular thyroid tumors. RAS mutations are found in follicular adenomas but are more prevalent in follicular carcinomas (35, 36).
The US characteristics of autoimmune diseases include enlargement of the thyroid with reduced echogenicity, heterogeneity, and hypervascularity. Diffusely infiltrative papillary or follicular thyroid carcinomas may have all of these features and, thus, may be mistaken for autoimmune thyroid disease. Furthermore, patients may present with misleading thyrotoxicosis (Fig. 12).
Ultrasonography findings indicative of diffuse thyroid disease (DTD) are characterized by a diffusely enlarged thyroid gland, decreased or increased diffuse heterogeneous parenchymal echogenicity, a coarse echotexture, micronodulation, and scattered microcalcifications (37, 38).
Ultrasonography is not generally required to diagnose DTD; however, Hashimoto's thyroiditis is primarily a subclinical disease, and US can detect this subset of patients before they come to clinical attention when typical US findings are present (39). US plays a role excluding focal thyroid disease and in assessing the size of the thyroid gland. US is also used to distinguish, characterize, and detect diffuse infiltrating tumors (37, 39).
Hyperthyroidism is rare in childhood and is most commonly caused by Graves' disease. It affects 0.02% of children or 1 in 5000 (23). The peak incidence occurs from 11-15 years of age with a female predominance. A positive family history is common (37). Antibodies stimulate TSHR leading to lymphocyte infiltration of the thyroid gland. US reveals diffuse heterogeneous and hypoechoic enlargement of the thyroid (Fig. 13). Color Doppler imaging reveals a hypervascular pattern in the thyroid referred to as a "thyroid inferno".
Hashimoto's thyroiditis is one of the most common organ-specific autoimmune diseases. Its annual incidence is estimated to be between 0.3 and 1.5 cases per 1000 persons, with no significant race-related predominance (37). Hashimoto's thyroiditis affects 1.3% of children and has a female predominance (23, 37, 38). This disease, which is the most common DTD, is characterized by diffuse lymphocytic infiltration. Diagnosis is made by detecting antithyroid antibodies, including antithyroid peroxidase and antithyroglobulin antibodies. US is useful for measuring thyroid size and assessing echotexture. US imaging shows an enlarged gland with a diffusely heterogeneous, coarse echotexture and multiple discrete hypoechoic micronodules ranging from 1-6 mm in diameter. There may be coarse septations from fibrous bands (37, 38).
Acute suppurative thyroiditis is rare and is the result of microbial infection of the thyroid gland. Patients present with a tender mass or swelling over the thyroid gland and fever. When the left lobe of the thyroid is involved and a fistula is present between this lobe and the ipsilateral pyriform sinus, the possibility of a left third pharyngeal pouch remnant should be considered (Fig. 14). The infected portion of the gland appears enlarged and heterogeneous on US scans. A focal abscess may have developed, and prominent swelling in adjacent soft tissue resulting from associated myositis and cellulitis may be present (18, 23, 37).
Imaging, particularly US, plays an important role evaluating the various thyroid diseases affecting pediatric patients. Dual imaging with US and scintigraphy increases the diagnostic yield for CH. US is the most sensitive diagnostic modality for evaluating thyroid nodules and to diagnose of thyroid carcinoma because it facilitates aspiration biopsies and follow-up. Testing for cancer-specific mutations in thyroid FNA samples increases the diagnostic accuracy of FNA cytology. Additionally, US enables the diagnosis of lymph node metastasis.
Figures and Tables
 | Fig. 1Normal thyroid development.
A. Thyroid develops from median endodermal thickening in floor of primordial pharynx, between first and second pharyngeal arches. Thickening rapidly leads to formation of small outpouching referred to as thyroid primordium. B. Thyroid primordium is initially hollow but quickly becomes solid and divides into right and left lobes. C. Developing thyroid gland descends in neck. Thyroid gland is connected for short time to tongue by narrow tube (i.e., thyroglossal duct). By seventh week it assumes definitive shape and reaches its final site within neck.
|
 | Fig. 2Thyroid aplasia in 29-day-old boy.Thyroid stimulating hormone level was > 95 µU/mL (normal range, 0.25-4.0 µU/mL). A. Transverse ultrasonography (US) reveals hyperechogenic structures (arrows) on both sides of trachea in empty thyroid area, signifying remnants of ultimobranchial body. Refer to Figure 4A for US findings of normal thyroid gland. B. Anteroposterior views of Tc-99m scan confirm absence of detectable thyroid activity, with only background soft tissue and salivary glands visible.
|
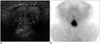 | Fig. 3Ectopic thyroid gland in 29-day-old boy.Thyroid stimulating hormone level was 40.66 µU/mL. A. Solid nodular mass (arrows), similar to normal thyroid echogenicity, was present in anterior strap muscle at level of larynx (arrowheads, cricoarytenoid joint) on transverse scan. B. Image of Tc-99m scan shows isotope uptake in upper neck with no uptake in normal thyroid gland.
|
 | Fig. 4Transient hypothyroidism in 1-month-old boy born at 36 weeks and weighing 2440 g.Neonatal screening revealed abnormally high thyroid stimulating hormone (TSH) (27.65 µU/mL) and free thyroxine (T4) (1.76 ng/dL; normal range, 0.70-2.0 ng/dL) levels. A. Transverse ultrasonography scan shows normal thyroid gland. B. Scintigraphy scan obtained on same day shows no visible thyroid activity. C. Follow-up scintigraphy at 1 year shows normal thyroid uptake. TSH and free T4 levels were normal. Patient discontinued levothyroxine treatment.
|
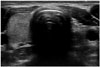 | Fig. 5Hemiagenesis in 17-year-old male incidentally detected during pneumothorax evaluation. Transverse ultrasonography revealed no left thyroid gland. Thyroid function was normal. |
 | Fig. 6Dyshormonogenesis in 15-day-old female.
A. Transverse ultrasonography scan shows enlarged thyroid gland. B. Tc-99m scintigraphy shows enlarged thyroid gland with increased uptake (20%; normal range, 2-4%). Thyroid stimulating hormone level at time of initial study was > 60 µU/mL, and free thyroxine level was 0.12 ng/dL (normal range, 0.89-1.7 ng/dL).
|
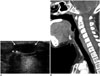 | Fig. 7Thyroglossal duct cyst in 8-year-old girl presenting with palpable mass in anterior neck.Longitudinal (A) and sagittal (B) ultrasonography revealed well-defined cystic lesion (long arrows) below hyoid bone (arrowheads).
|
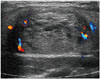 | Fig. 8Longitudinal scan shows well defined oval isoechoic nodule with inner cystic changes in 18-year-old female.Nodular hyperplasia was confirmed by hemithyroidectomy.
|
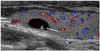 | Fig. 9Longitudinal scan shows colloid cyst with comet tail artifact (arrow) in 18-year-old female who underwent neck ultrasonography to evaluate cervical lymphadenopathy. |
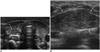 | Fig. 10Intrathyroid thymus in 5-month-old boy who underwent ultrasonography (US) for cervical lymphadenopathy.
A. Transverse US scan shows hypoechoic nodular lesion with inner echogenic strands and dots, similar to normal thymus in mediastinum (arrows) (B).
|
 | Fig. 1113-year-old female presenting with palpable mass.Transverse ultrasonography scan shows isoechoic oval solid nodule (arrows). Follicular adenoma was confirmed by hemithyroidectomy.
|
 | Fig. 12Papillary thyroid cancer presenting with hyperthyroidism in 12-year-old female.Longitudinal ultrasonography scan (A) shows oval hyperechoic nodule with numerous microcalcifications (arrows) and multiple metastatic lymphadenopathy in left thyroid at levels II-IV (B). Microcalcifications and cystic changes are seen. C. Tc-99m scintigraphy shows increased uptake (thyroid uptake 7.2%; normal range, 2-4%), diffuse enlargement of both thyroid gland lobes, and increased uptake in thyroid nodule (short arrows) and in left lateral neck due to metastasis to lymph nodes (long arrows). Surgery confirmed papillary thyroid cancer with metastatic lymphadenopathy and underlying lymphocytic thyroiditis.
|
 | Fig. 13Grave's disease in 17-year-old female.
A. Transverse ultrasonography scan shows marked enlargement of thyroid gland and inhomogeneous decrease in echogenicity. B. Color Doppler image shows 'thyroid inferno' pattern of hypervascularity.
|
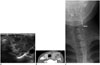 | Fig. 14Suppurative thyroiditis in 10-year-old boy who presented with palpable mass with erythema and tenderness in left side of neck for 3 days.
A. Transverse ultrasonography scan shows ill-defined, heterogeneous, hypoechoic lesion (arrows) in left lobe of thyroid gland. B. Contrast computed tomography scan shows ill-defined low-density lesion (arrows) in left lobe with surrounding inflammation. C. Esophagogram shows pyriform sinus fistula (arrow) that was cauterized with 5% trichloroacetic acid.
|
Table 1
US Findings and Additional Studies of Thyroid Nodules

References
1. Donaldson M, Jones J. Optimising outcome in congenital hypothyroidism; current opinions on best practice in initial assessment and subsequent management. J Clin Res Pediatr Endocrinol. 2013; 5:Suppl 1. 13–22.
2. Perry RJ, Maroo S, Maclennan AC, Jones JH, Donaldson MD. Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Arch Dis Child. 2006; 91:972–976.
3. Chang YW, Lee DH, Hong YH, Hong HS, Choi DL, Seo DY. Congenital hypothyroidism: analysis of discordant US and scintigraphic findings. Radiology. 2011; 258:872–879.
4. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006; 13:427–453.
5. Som PM, Smoker WR, Reidenberg JS, Bergemann AD, Hudgins PA, Laitman J. Embryology and anatomy of the neck. In : Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis, MO: Mosby;2011. p. 2117–2163.
6. Zander DA, Smoker WR. Imaging of ectopic thyroid tissue and thyroglossal duct cysts. Radiographics. 2014; 34:37–50.
7. Yoon HC, Kim NC, Lee DH. A cost-benefit analysis on neonatal screening of phenylketonuria and congenital hypothyroidism in Korea. Korean J Pediatr. 2005; 48:369–375.
8. Lee ST, Lee DH, Kim JY, Kwon MJ, Kim JW, Hong YH, et al. Molecular screening of the TSH receptor (TSHR) and thyroid peroxidase (TPO) genes in Korean patients with nonsyndromic congenital hypothyroidism. Clin Endocrinol (Oxf). 2011; 75:715–721.
9. Macchia PE. Recent advances in understanding the molecular basis of primary congenital hypothyroidism. Mol Med Today. 2000; 6:36–42.
10. Korzeniewski SJ, Grigorescu V, Kleyn M, Young WI, Birbeck G, Todem D, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr. 2013; 162:177–182.
11. Mitchell ML, Hsu HW, Sahai I. Massachusetts Pediatric Endocrine Work Group. The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf). 2011; 75:806–810.
12. Davy T, Daneman D, Walfish PG, Ehrlich RM. Congenital hypothyroidism. The effect of stopping treatment at 3 years of age. Am J Dis Child. 1985; 139:1028–1030.
13. Jeon G, Shin JH, Hong HS, Kim JH, Hwang JH, Goo DE, et al. Sonographic measurement of normal thyroid gland in the neonates. J Korean Soc Med Ultrasound. 2001; 20:207–212.
14. Ueda D. Normal volume of the thyroid gland in children. J Clin Ultrasound. 1990; 18:455–462.
15. Perry RJ, Hollman AS, Wood AM, Donaldson MD. Ultrasound of the thyroid gland in the newborn: normative data. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F209–F211.
16. Vade A, Gottschalk ME, Yetter EM, Subbaiah P. Sonographic measurements of the neonatal thyroid gland. J Ultrasound Med. 1997; 16:395–399.
17. Recommended normative values for thyroid volume in children aged 6-15 years. World Health Organization & International Council for Control of Iodine Deficiency Disorders. Bull World Health Organ. 1997; 75:95–97.
18. Som PM, Smoker WR, Curtin HD, Reidenberg JS, Laitman J. Congenital lesions of the neck. In : Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis, MO: Mosby;2011. p. 2235–2285.
19. Khurana KK, Labrador E, Izquierdo R, Mesonero CE, Pisharodi LR. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents, and young adults: a multi-institutional study. Thyroid. 1999; 9:383–386.
20. Avula S, Daneman A, Navarro OM, Moineddin R, Urbach S, Daneman D. Incidental thyroid abnormalities identified on neck US for non-thyroid disorders. Pediatr Radiol. 2010; 40:1774–1780.
21. Hung W, Anderson KD, Chandra RS, Kapur SP, Patterson K, Randolph JG, et al. Solitary thyroid nodules in 71 children and adolescents. J Pediatr Surg. 1992; 27:1407–1409.
22. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14.
23. Babcock DS. Thyroid disease in the pediatric patient: emphasizing imaging with sonography. Pediatr Radiol. 2006; 36:299–308. quiz 372-373.
24. Chang YW, Hong HS, Choi DL. Sonography of the pediatric thyroid: a pictorial essay. J Clin Ultrasound. 2009; 37:149–157.
25. Megremis S, Stiakaki E, Tritou I, Bonapart IE, Tsilimigaki A. Ectopic intrathyroidal thymus misdiagnosed as a thyroid nodule: sonographic appearance. J Clin Ultrasound. 2008; 36:443–447.
26. Korea Central Cancer Registry Ministry of Health and Welfare. Republic of Korea. 2011 Annual report of the Korea. Central Cancer Registry. Seoul: Ministry of Health and Welfare Republic of Korea;2014. Accessed April 2004. Web site. http://www.ncc.re.kr/.
27. Grigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002; 95:724–729.
28. Brink JS, van Heerden JA, McIver B, Salomao DR, Farley DR, Grant CS, et al. Papillary thyroid cancer with pulmonary metastases in children: long-term prognosis. Surgery. 2000; 128:881–886. discussion 886-887.
29. Shin LK, Olcott EW, Jeffrey RB, Desser TS. Sonographic evaluation of cervical lymph nodes in papillary thyroid cancer. Ultrasound Q. 2013; 29:25–32.
30. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000; 85:1170–1175.
31. Lee KY, Hong HS, Lee EH, Yi BH, Lee HK, Lee YW, et al. Imaging and clinical features of thyroid cancer in children and adolescents. J Korean Soc Radiol. 2011; 65:181–189.
32. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–262.
33. Yang JY, Hong HS, Lee EH, Kim CH, Kwak JJ, Lee SW, et al. Analysis of the BRAFV600E mutation in thyroid nodules: the preoperative diagnostic role of fine-needle aspiration biopsy for patients with papillary thyroid cancer and its impact on patient care. J Korean Soc Ultrasound Med. 2011; 30:103–112.
34. Grebe SK, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995; 24:761–801.
35. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003; 88:2318–2326.
36. Volante M, Rapa I, Gandhi M, Bussolati G, Giachino D, Papotti M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab. 2009; 94:4735–4741.
37. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003; 348:2646–2655.
38. Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med. 1996; 15:813–819.
39. Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000; 10:251–259.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download