Abstract
Objective
Materials and Methods
Results
Conclusion
Figures and Tables
Fig. 1
End-systole (ES) and end-diastole (ED) cine MR images in 54-year-old man with hypertrophic cardiomyopathy. Trabeculae are hypertrophied and abundant, and are difficult to discern from compact myocardium at ES phase.
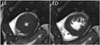
Fig. 2
Representative example of endocardial contouring using three methods in 51-year-old man with hypertrophic cardiomyopathy. Method A indicates classic method for inclusion of trabeculae in left ventricular (LV) cavity volume. Single short-axis-view true FISP MR images at end-diastolic (ED) and end-systolic (ES) phases show inclusion of trabeculae in LV cavity volume. Method B includes closely adjacent trabeculae in LV cavity volume. ES image shows inclusion of trabeculae in LV cavity volume similar to method A. However, endocardial contour is smaller, based solely on cavity and closely adjacent trabeculae. Method C indicates classic method for exclusion of trabeculae from LV cavity volume. Trabeculae were completely excluded from cavity volume.
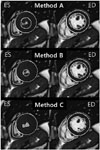
Fig. 3
Bar graphs showing mean values of left ventricular measures using three different methods.

Table 1
Comparison of LV Measurements According to Three Different Methods of Delineation at MR Imaging in 20 Patients with HCM

Note.-*Date are presented as least-square means ± standard error, †Post hoc analysis using Linear Mixed Effect Model and adjusted p value according to Sidak correction for multiple testing. EDV = end-diastolic volume, EF = ejection fraction, ESV = end-systolic volume, HCM = hypertrophic cardiomyopathy, LV = left ventricular, SV = stroke volume
Table 2
Agreement of LV Stroke Volume with Forward Flow of Ascending Aorta as Reference in Each Method

Note.-*Bland-Altman analysis, †Wilcoxon signed-rank test, ‡Friedman test for comparison of absolute percentage errors among three methods and Scheffe's correction for multiple post-hoc analysis. Significant differences in absolute percentage errors between methods A and B, and between methods B and C (all, p < 0.001). SD = standard deviation
Table 3
Comparison of LV Mass between ES and ED Phases in All Three Methods

Table 4
Interobserver Reproducibility of LV Measurements According to Three Different Methods of Delineation in 20 Patients with HCM





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download