Abstract
Objective
To evaluate the technical aspects and outcomes of endovascular recanalization of a thrombosed native arteriovenous fistula (AVF) complicated with an aneurysm.
Materials and Methods
Sixteen patients who had a thrombosed AVF complicated with an aneurysm (two radiocephalic and 14 brachiocephalic) were included in this study. Recanalization procedures were performed by mechanical thrombectomy using the Arrow-Trerotola percutaneous thrombectomy device and adjunctive treatments. We evaluated dose of thrombolytic agent, underlying stenosis, procedure time, technical and clinical success, and complications. The primary and secondary patency rates were calculated using the Kaplan-Meier analysis.
Results
The thrombolytic agents used were 100000 U urokinase mixed with 500 IU heparin (n = 10) or a double dose of the mixture (n = 6). The thrombi in aneurysms were removed in all but two patients with non-flow limiting residual thrombi. One recanalization failure occurred due to a device failure. Aspiration thrombectomy was performed in 87.5% of cases (n = 14). Underlying stenoses were found in the outflow draining vein (n = 16), arteriovenous anastomosis or juxtaanastomosis area (n = 5), and the central vein (n = 3). Balloon angioplasty was performed for all stenoses in 15 patients. Two patients with a symptomatic central vein stenosis underwent insertion of a stent after balloon angioplasty. Mean procedure time was 116.3 minutes. Minor extravasation (n = 1) was resolved by manual compression. Both technical and clinical success rates were 93.8% (n = 15). The primary patency rates at 3, 6, and 12 months were 70.5%, 54.8%, and 31.3%, respectively. The secondary patency rates at 3, 6, and 12 months were 70.5%, 70.5%, and 47.0%, respectively.
A native arteriovenous fistula (AVF) has been the choice for primary vascular access due to lower infection rates and longer durability for hemodialysis in patients with end stage renal disease (1). In recent decades, the Kidney Disease Outcomes Quality Initiative has reported that native AVF is preferred over other forms of vascular access due to the lower rate of complications (2, 3). However, the blood vessel resources available to create an AVF are quite limited. In addition, AVF can be dysfunctional due to various complications. Among these complications, thrombosis is commonly encountered in clinical practice and one of the major causes of eventual AVF failure (4).
The incidence of thrombosis in patients with an AVF is lower than that in patients with a prosthetic arteriovenous graft (AVG). However, unlike thrombosis of an AVG, AVF usually has a large thrombus burden because it is commonly accompanied by a large aneurysm or aneurysmal degeneration (5, 6, 7). If the thrombus burden is not heavy, the case can be easily treated by endovascular methods. However, recanalization becomes difficult with an AVF containing a large clot in an aneurysm, due to the dilated lumen and old layers of wall adherent thrombi that can cause a significant pulmonary embolism (6, 8). For these reasons, endovascular recanalization is contraindicated in cases of large aneurysms or aneurysmal degeneration (6). However, if the thrombosed AVF is routinely abandoned, most patients will exhaust the more desirable upper extremity access and eventually be prohibited from further hemodialysis (9). Therefore, salvage of thrombosed vascular access is essential to decrease morbidity and mortality in patients undergoing hemodialysis. The purpose of this study was to evaluate the technical aspects and outcomes of endovascular recanalization of thrombosed AVFs complicated with an aneurysm.
Our Institutional Review Board approved this retrospective study with a waiver of the requirement for patient informed consent. We reviewed our hospital's electronic medical records for patients who underwent endovascular treatment for a malfunctioning AVF between January 2011 and December 2013. During the study period, 52 patients who had a thrombosed AVF underwent endovascular recanalization. Among these, 16 who had a thrombosed AVF complicated with an aneurysm (eight men and eight women; mean age ± standard deviation, 63.2 ± 15.4) were included in this study. The types of AVFs were radiocephalic (n = 2) and upper-arm brachiocephalic (n = 14). The ages of the AVFs at the time of endovascular recanalization were 2.5-65 months (mean, 28.5 ± 18.5 months). Two patients had a history of angioplasty due to stenosis of a fistula prior the AVF thrombosis (Table 1).
Informed consent was obtained before initiating treatment. Intravenous access was established with an 18-gauge intravenous catheter. No prophylactic antibiotic was administered. Physical and ultrasound (US) examinations were performed to evaluate the degree of thrombosis and the location and size of the aneurysm. Local anesthesia was provided at the puncture site with 1% lidocaine hydrochloride. The puncture site and direction were determined based on thrombosis location, as one of the following types: 1) single antegrade puncture near the arteriovenous (AV) anastomosis site directed toward the central vein when the thrombosis was distal to the AV anastomosis site, 2) single retrograde puncture of a patent segment of draining vein directed toward the AV anastomosis site when the thrombosis was near the AV anastomosis site, and 3) double bidirectional puncture of the thrombosed segment when the thrombosis was total. The aneurysmal segment was not used as a puncture site. An antegrade puncture was performed initially when a double bidirectional puncture was required. A retrograde puncture was performed after recanalizing central or distal to the AV anastomosis site. All punctures were performed with a micropuncture set (Cook Medical Inc., Bloomington, IL, USA) under US guidance without a tourniquet.
After successfully inserting a 7-Fr (2-inch) introducer sheath, patients received 3000 IU heparin intravenously. In all cases, a 5-Fr angiographic catheter (Cook Medical) and a 0.035-inch angled guidewire (Terumo, Tokyo, Japan) were used to pass the thrombosed segment. When the angiographic catheter was advanced successfully into the non-thrombosed segment or artery, an adjunctive thrombolytic agent was infused into the thrombus through the angiographic catheter to mobilize and fragment the thrombus. According to the thrombus burden, 100000 U urokinase (UK) mixed with 500 IU heparin or a double dose of the mixture was used. After changing the guidewire to a 0.025-inch wire (Terumo), a mechanical thrombectomy was performed with the Arrow-Trerotola percutaneous thrombectomy device (PTD) (Arrow, Reading, PA, USA). The aneurysm was manually compressed or squeezed to facilitate fragmentation of thrombi by increasing the contact area between the PTD basket and the thrombus because the activated PTD basket could not cover the thrombus in the aneurysm due to the dilated lumen. An aspiration thrombectomy was also conducted using a 6- or 7-Fr soft-tip guiding catheter.
Balloon angioplasty was performed for an underlying stenosis in the draining vein and the anastomosed or juxta-anastomosed area until all stenotic segments were sufficiently dilated (< 30% residual stenosis) and brisk flow was reestablished through the fistula. Balloon size was determined according to the size of the adjacent normal segment. A completion angiogram was obtained from the AV anastomosis to the superior vena cava. Angioplasty was performed within 2 weeks when a central vein stenosis was detected. A stent was inserted when a residual stenosis or persistent collateral veins were detected after balloon angioplasty. Hemostasis was performed with manual compression after the procedures in all cases.
An aneurysm was defined as dilation of more than twice the normal segment of the outflow vein or dilation > 3 cm in diameter. We categorized the degree of thrombosis as ≥ 50% or < 50%. Among ≥ 50% thrombosis cases, total thrombosis was defined when the thrombosis occurred from the AV anastomosis site to the cephalic arch for a brachiocephalic fistula, or from the AV anastomosis site to the antecubital fossa level for a radiocephalic fistula.
We applied the Society of Interventional Radiology reporting standards (10). Anatomic success was defined as < 30% residual diameter stenosis after treatment. Clinical success after treating thrombosed access was defined as resumption of normal dialysis for at least one session. Primary patency was defined as the interval following intervention until the next access thrombosis or repeated intervention. Secondary patency was defined as the interval after intervention until the access site was surgically declotted, revised, or was abandoned because of inability to treat the original lesion, choice of surgeon, transplant, or loss to follow-up. Complications were categorized as major or minor. Major complications included those requiring therapy or hospitalization, causing permanent adverse sequelae, or death. Minor complications were those requiring no or nominal therapy without consequences.
Patient information was censored from analyses at the time of last known follow-up. A Kaplan-Meier analysis was used to calculate primary and secondary patency. All analyses were performed with Statistical Package for the Social Sciences (SPSS) ver. 20 software (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered significant.
Greater than 50% AVF thrombosis was detected in 15 of 16 (93.8%) patients, including 13 brachiocephalic and two radiocephalic AVFs (Fig. 1). Only one (6.2%) patient with a brachiocephalic AVF had < 50% thrombosis. Of patients with ≥ 50% thrombosis, total thrombosis occurred in 12 (75%), including 10 brachiocephalic and two radiocephalic AVFs. According to the thrombosis site, single antegrade, single retrograde, and double bidirectional punctures were performed in two, four, and 10 patients, respectively.
An adjunctive thrombolytic agent was used in all patients. The dose of thrombolytic agent used was 100000 U UK mixed with 500 IU heparin in 10 patients and 200000 U UK mixed with 1000 IU heparin in six patients with a larger clot burden. Thrombectomy was performed with a PTD after infusing the adjunctive thrombolytic agent. The thrombi in the aneurysm were completely removed using the PTD in all except two patients with non-flow limiting residual thrombi. One patient with residual thrombi had a dead space where the PTD could not reach despite external manipulation. The other patient with residual thrombi experienced device failure, and chronic wall adherent thrombi could not be removed effectively. However, no further intervention was performed because there was a no flow limitation on fistulography.
Two device failures occurred during thrombectomy for the aneurysmal segment. The shaft of the PTD kinked during the operation in one patient, and the inner wire was elongated, resulting in a PTD device stuck in the introducer sheath. We cut the elongated PTD wire with wire cutters and performed en bloc removal of the remnant PTD in the AVF with the introducer sheath through the puncture site. Then, we completed the procedure with another new PTD and restored flow despite a residual thrombus (Fig. 2). In the other patient, the PTD basket was completely severed. We removed the distal end of the PTD with a snare catheter. However, the patient refused completion of the recanalization procedure.
After fragmenting the thrombus, we performed aspiration thrombectomy using a 6-Fr or 7-Fr soft tip guiding catheter in 14 patients. An aspiration thrombectomy was not needed in one patient after mechanical thrombectomy using a PTD.
Underlying stenosis was detected in the outflow draining vein (n = 16), AV anastomosis or juxta-anastomosis area (n = 5), and the central vein (n = 3). A cephalic arch stenosis was detected in 10 patients. Stenoses in the outflow draining vein were multifocal. Balloon angioplasty was performed for the stenotic segments after removing clots in 15 patients. Balloon size ranged from 5 to 7 mm. All outflow draining vein stenoses were successfully dilated after balloon angioplasty except two cephalic arch stenoses showing mild residual stenosis. However, no further angioplasty or stent procedures were performed for residual cephalic arch stenoses because there was no flow limitation on fistulography.
The mean procedure time from initial puncture to completion of fistulography was 116.3 minutes (standard deviation = 69.0 minutes). Minor extravasation was resolved in one patient by manual compression.
Early rethrombosis occurred in one patient with a residual cephalic arch stenosis on day 4 after the interventional procedure despite two sessions of successful dialysis. We tried to recanalize the AVF, but could not pass the guidewire through the thrombosed cephalic arch. We experienced perforation of the cephalic arch, and contrast extravasation hindered continuing infusion of the thrombolytic agent.
A left innominate vein stenosis was detected in two patients, whereas a right innominate vein stenosis was detected in one patient. A mild in-stent stenosis was found in a patient who underwent insertion of a stent into the left innominate vein. The patient had no symptoms related to the stenosis. Angioplasty was performed 6 and 12 days later for two patients with a symptomatic central vein stenosis, respectively. Stents were inserted after balloon angioplasty because there were significant residual stenoses and persistent collateral veins. The diameters of the stents used were 12 and 14 mm, respectively.
Both technical and clinical success rates of recanalization were 93.8% (15 of 16 patients). One technical failure occurred due to a device failure. The primary patency rates at 3, 6, and 12 months were 70.5%, 54.8%, and 31.3%, respectively (Fig. 3). Secondary patency rates at 3, 6, and 12 months were 70.5%, 70.5%, and 47.0%, respectively (Fig. 4, Table 2).
An aneurysm is not a rare complication of AVF and incidence is up to 30% of AVFs (11). According to some reports, an aneurysm is defined as a fusiform or saccular dilatation of the outflow vein whose diameter is greater than twice the normal caliber of the adjacent normal segment (12). An uncomplicated aneurysm can be managed without cannulation (3). However, there is always the possibility of a complication, such as thrombosis, skin breakdown, bleeding, or poor flow resulting in inadequate dialysis. Among these complications, thrombosis is the most frequently encountered etiology for AVF dysfunction. It is one of the main causes of abandoning an aneurysmal AVF (13).
Aneurysmal AVFs are prone to thromboses due to the turbulent flow in an aneurysm (14, 15). If they thrombose, they usually have more thrombi than AVFs not complicated with an aneurysm (6). Thus, recanalizing AVFs with a large aneurysm always carries a risk for a fatal iatrogenic pulmonary embolism. Thrombi in aneurysms are usually old, organized, and firmly attached to the vessel wall because they have been formed over a long period of time (6). These characteristics usually make recanalization procedures difficult, resulting in abandoning the AVF and creating a new AVF or AVG. However, vascular resources for creating an AVF are quite limited. Less desirable alternative sites, such as the leg or chest wall, are used in some patients. All available sites may be used in some patients, prohibiting further hemodialysis (9). Therefore, salvage of a thrombosed AVF is important to decrease morbidity and mortality in patients undergoing hemodialysis.
Several authors have treated thrombosed AVFs using various thrombectomy devices such as a PTD, rotating pigtail catheter, and rheolytic or flow-based devices (9, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). The technical or clinical success rates in these studies were 73-100%. However, aneurysmal AVFs are mentioned only in a very small portion of cases. Therefore, the success rates of thrombosed aneurysmal AVFs cannot be evaluated. Our study showed the success and patency rates for endovascular treatment of thrombosed aneurysmal AVFs because all of our cases were aneurysmal AVFs.
A considerable number of thrombi can remain due to a large aneurysmal vein despite use of various thrombectomy devices, which can result in technical or clinical failure. We achieved a technical and clinical success rate of 93.8% (15/16) and ≥ 50% thrombosis of a fistula was detected in 93.8% (15/16) of patients. We mainly used a PTD for declotting, whereas we applied an external maneuver, such as compression or squeezing, to contact the rotational basket of the thrombectomy device with clots while declotting the aneurysmal portion. These maneuvers removed most clots in the aneurysm, which resulted in a high technical and clinical success rate.
Attempts to recanalize aneurysmal or thrombosed AVFs have been made using venotomy (5, 7). These new methods resulted from the difficulties recanalizing aneurysmal AVFs, as conventional endovascular methods show high technical or clinical failure due to a dilated lumen and old wall attached thrombi. This procedure typically consists of proximal and distal occlusion of the venotomy site with a balloon, venotomy, manual propulsion of the thrombi, and suturing of the venotomy site. In one study using this method, 69 venotomy and manual propulsion procedures were performed in 56 patients. In that study, the technical and clinical success rates were 95.7% and 91.3%, respectively (7). However, only a few studies have used this method. Therefore, further clinical data must be accumulated for routine use of this method.
We experienced two device failures due to kinking of inner PTD wire during thrombectomy procedures for the aneurysmal segment. Irregular rotation of the PTD in the dilated aneurysmal segment may have caused the kinking, as the PTD has a relatively thin 0.025-inch guidewire system. Therefore, the guidewire should be sufficiently advanced into the distal portion of the aneurysm to firmly support and prevent irregular rotation of the PTD during thrombectomy of the aneurysmal segment. One report discussed disconnection of the rubber PTD tip as a device-related complication during thrombectomy for a hemodialysis fistula. Five cases of a rubber tip disconnection were reported among 453 thrombectomies. Those authors experienced a disconnected rubber tip when they activated a PTD within an acute angulated vessel or an indwelled stent (26). Therefore, careful use of a PTD is highly recommended because there can be complications during thrombectomy and reuse of the device is not recommended.
Rocek et al. (22) described results from 10 thrombectomy cases for AVF using a PTD. Their primary patency rates at 3 and 6 months were 70% and 60%, respectively, and the assisted primary patency rates were 80% and 80%, respectively. Shatsky et al. (18) reported 62 thrombectomy procedures including two aneurysmal AVF with use of a PTD. Primary patency rates at 6 and 12 months were 38% and 18%, respectively, and secondary patency rates were 74% and 69%, respectively. Our results show similar primary patency rates and slightly lower assisted primary or secondary patency rates compared with those of previous studies. However, our 12 month primary patency rate was relatively low, whereas the secondary patency rate increased to 47%. These results suggest that the function of aneurysmal AVFs can be easily impaired regardless of high technical or clinical success rates of the recanalization procedure. However, the lifespan of a aneurysmal AVF can be prolonged by additional interventional treatment once recanalization succeeded. Therefore, we strongly believe that the salvage procedure for a thrombosed aneurysmal AVF is quite important for patients undergoing dialysis in terms of saving available vessels for future use. In addition, it saved time, rather than creating a new AVF or AVG.
Our study had limitations due to its retrospective nature and small sample size. Therefore, a larger prospective randomized study is needed to evaluate the technical and clinical success and patency rates more accurately.
In conclusion, a thrombosed AVF complicated with an aneurysm was successfully recanalized and secondary patency was prolonged with endovascular treatment.
Figures and Tables
 | Fig. 186-year-old woman with radiocephalic arteriovenous fistula (AVF) in left forearm vein.
A. Fistulogram obtained through retrograde puncture of draining vein shows > 50% thrombosis in draining vein. Saccular (white arrow) and diffuse aneurysms (black arrow) are shown on fistulogram. B. Mechanical thrombectomy was performed with percutaneous thrombectomy device to fragment thrombi. C. After aspiration thrombectomy and balloon angioplasty for stenotic segment. Catheter was advanced into arteriovenous anastomosis site to complete fistulogram. Fistulogram showing that flow through radiocephalic AVF was restored without limitation and without residual thrombi.
|
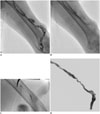 | Fig. 257-year-old man with brachiocephalic arteriovenous fistula in left upper arm.
A. Fistulogram obtained through retrograde puncture of draining vein showing > 50% thrombosis and multifocal aneurysms in draining vein. B. Inner wire of percutaneous thrombectomy device (PTD) kinked during thrombectomy for aneurysmal segment. C. Remnant PTD was removed through puncture site after cutting inner wire with wire cutters. D. Recanalization procedures were resumed with new PTD device. Flow was restored on completed fistulogram, despite residual thrombi.
|
Table 1
Patients Characteristics

| Parameter | Value |
|---|---|
| No. of patients | 16 |
| Sex (M/F) | 8/8 |
| Mean age (year) | 63.2 ± 15.4 |
| Fistula type | |
| Radiocephalic | 2 |
| Brachiocephalic | 14 |
| Mean age of AVF at procedure (month) | 28.5 ± 18.5 |
Table 2
Results of Endovascular Recanalization

References
1. Kalman PG, Pope M, Bhola C, Richardson R, Sniderman KW. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999; 30:727–733.
2. NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997; 30:4 Suppl 3. S150–S191.
3. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006; 48:Suppl 1. S176–S247.
4. Fan PY, Schwab SJ. Vascular access: concepts for the 1990s. J Am Soc Nephrol. 1992; 3:1–11.
5. Joo SM, Kim HC, Min SI, Hur S, Jae HJ, Chung JW, et al. Recanalization of thrombosed arteriovenous fistulas for hemodialysis by minimal venotomy. J Vasc Interv Radiol. 2013; 24:401–405.
6. Turmel-Rodrigues L. Application of percutaneous mechanical thrombectomy in autogenous fistulae. Tech Vasc Interv Radiol. 2003; 6:42–48.
7. Won JH, Bista AB, Bae JI, Oh CK, Park SI, Lee JH, et al. A venotomy and manual propulsion technique to treat native arteriovenous fistulas occluded by thrombi. AJR Am J Roentgenol. 2012; 198:460–465.
8. Bent CL, Sahni VA, Matson MB. The radiological management of the thrombosed arteriovenous dialysis fistula. Clin Radiol. 2011; 66:1–12.
9. Hyun JH, Lee JH, Park SI. Hybrid surgery versus percutaneous mechanical thrombectomy for the thrombosed hemodialysis autogenous arteriovenous fistulas. J Korean Surg Soc. 2011; 81:43–49.
10. Gray RJ, Sacks D, Martin LG, Trerotola SO. Society of Interventional Radiology Technology Assessment Committee. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S433–S442.
11. Salahi H, Fazelzadeh A, Mehdizadeh A, Razmkon A, Malek-Hosseini SA. Complications of arteriovenous fistula in dialysis patients. Transplant Proc. 2006; 38:1261–1264.
12. Rajput A, Rajan DK, Simons ME, Sniderman KW, Jaskolka JD, Beecroft JR, et al. Venous aneurysms in autogenous hemodialysis fistulas: is there an association with venous outflow stenosis. J Vasc Access. 2013; 14:126–130.
13. Pasklinsky G, Meisner RJ, Labropoulos N, Leon L, Gasparis AP, Landau D, et al. Management of true aneurysms of hemodialysis access fistulas. J Vasc Surg. 2011; 53:1291–1297.
14. Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Steady flow in an aneurysm model: correlation between fluid dynamics and blood platelet deposition. J Biomech Eng. 1996; 118:280–286.
15. Peattie RA, Asbury CL, Bluth EI, Riehle TJ. Steady flow in models of abdominal aortic aneurysms. Part II: Wall stresses and their implication for in vivo thrombosis and rupture. J Ultrasound Med. 1996; 15:689–696.
16. Rousseau H, Sapoval M, Ballini P, Dube M, Joffre F, Gaux JC, et al. Percutaneous recanalization of acutely thrombosed vessels by hydrodynamic thrombectomy (Hydrolyser). Eur Radiol. 1997; 7:935–941.
17. Rajan DK, Clark TW, Simons ME, Kachura JR, Sniderman K. Procedural success and patency after percutaneous treatment of thrombosed autogenous arteriovenous dialysis fistulas. J Vasc Interv Radiol. 2002; 13:1211–1218.
18. Shatsky JB, Berns JS, Clark TW, Kwak A, Tuite CM, Shlansky-Goldberg RD, et al. Single-center experience with the Arrow-Trerotola Percutaneous Thrombectomy Device in the management of thrombosed native dialysis fistulas. J Vasc Interv Radiol. 2005; 16:1605–1611.
19. Cohen A, Korzets A, Neyman H, Ori Y, Baytner S, Belenky A, et al. Endovascular interventions of juxtaanastomotic stenoses and thromboses of hemodialysis arteriovenous fistulas. J Vasc Interv Radiol. 2009; 20:66–70.
20. Cho SK, Han H, Kim SS, Lee JY, Shin SW, Do YS, et al. Percutaneous treatment of failed native dialysis fistulas: use of pulse-spray pharmacomechanical thrombolysis as the primary mode of therapy. Korean J Radiol. 2006; 7:180–186.
21. Turmel-Rodrigues LA. Declotting a thrombosed Brescia-Cimino fistula by manual catheter-directed aspiration of the thrombus. Cardiovasc Intervent Radiol. 2005; 28:10–16.
22. Rocek M, Peregrin JH, Lasovicková J, Krajícková D, Slavíoková M. Mechanical thrombolysis of thrombosed hemodialysis native fistulas with use of the Arrow-Trerotola percutaneous thrombolytic device: our preliminary experience. J Vasc Interv Radiol. 2000; 11:1153–1158.
23. Vorwerk D, Schurmann K, Müller-Leisse C, Adam G, Bücker A, Sohn M, et al. Hydrodynamic thrombectomy of haemodialysis grafts and fistulae: results of 51 procedures. Nephrol Dial Transplant. 1996; 11:1058–1064.
24. Sahni V, Kaniyur S, Malhotra A, Fan S, Blakeney C, Fotheringham T, et al. Mechanical thrombectomy of occluded hemodialysis native fistulas and grafts using a hydrodynamic thrombectomy catheter: preliminary experience. Cardiovasc Intervent Radiol. 2005; 28:714–721.
25. Schmitz-Rode T, Wildberger JE, Hübner D, Wein B, Schürmann K, Günther RW. Recanalization of thrombosed dialysis access with use of a rotating mini-pigtail catheter: follow-up study. J Vasc Interv Radiol. 2000; 11:721–727.
26. Kim HM, Kim HC, Woo S, Son KR, Jae HJ. Disconnection of the rubber tip of arrow-trerotola percutaneous thrombolytic device. Korean J Radiol. 2014; 15:254–257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download