Abstract
Laparoscopic mini-gastric bypass surgery is a safe and simple surgical intervention for treating morbid obesity and diabetes mellitus and is now being performed more frequently. Radiologists must be critical in their postoperative evaluation of these patients. In this pictorial review, we explain and illustrate the surgical technique, normal postoperative anatomy, and associated complications as seen on imaging examinations, including fluoroscopy and computed tomography.
Obesity has become a worldwide pandemic health problem, and morbid obesity leads to a high rate of complications associated with metabolic syndrome, including type 2 diabetes mellitus (DM). There is strong evidence that bariatric surgery can successfully treat most patients with morbid obesity, and it is the only recommended effective treatment for morbid obesity (1, 2, 3). Furthermore, bariatric surgery, most typically gastric bypass, reduces DM-related deaths (4), which is why gastric bypass surgery has been proposed as a new treatment modality for type 2 DM (5, 6, 7).
Laparoscopic Roux-en-Y gastric bypass (LRYGB) is a safe and effective standard bariatric surgical procedure and has been performed recently to control DM (5, 8). Laparoscopic mini-gastric bypass is a safe alternative to LRYGB because of its simple surgical technique, comparable or lower complication rate, and similar efficacy, including weight reduction and control of DM (9, 10, 11, 12). However, mini-gastric surgery imaging findings have seldom been reported.
In this study, we explain and illustrate laparoscopic mini-gastric bypass surgery to control DM and the postoperative imaging features and associated complications as seen on fluoroscopy and computed tomography (CT) to familiarize radiologists.
Five or six trocars (four 12-mm and two 5-mm) are usually placed under general anesthesia. An endo-stapler (Echelon Flex Endoscopic Articulating Linear Cutter, Ethicon Endosurgery, Cincinnati, OH, USA) is used to divide the stomach from the angle of His to 2 cm proximal to the pylorus along the lesser curvature to form a long and narrow mini-gastric tube. Although the volume of the mini-gastric tube is usually 150-180 cc, it can be changed according to the patient's body mass index (BMI). Then, additional surgical clips are applied in a staple line to prevent post-procedural bleeding and as reinforcement.
The jejunum is mounted antecolically with the mini-gastric tube 200 cm distal to the Treitz ligament via a side-to-side anastomosis using an endo-stapler (Endo GIA Universal Stapler, Covidien Autosuture, Mansfield, MA, USA). The gastric antrum, duodenum, and proximal jejunum are consequently bypassed. An acute angle is made with an anchoring suture adjacent to the gastro-jejunostomy to prevent bile reflux and food in the bypassed segment (Fig. 1). A nasogastric tube is passed into the efferent loop after intraoperative leak testing and gastroscopy to detect intraluminal bleeding and to determine the size of the anastomotic site.
Our institution performs an upper gastrointestinal series (UGI) for all patients on postoperative day (POD) 1 with a water-soluble iodinated contrast agent to exclude obstructions, strictures, and staple line leaks before patients start a liquid diet. Diluted water-soluble contrast (up to 250 mL) is given via a straw. Fast-sequence images of the gastro-jejunostomy are obtained in anteroposterior, oblique, and lateral projections when the patient swallows. The shape and volume of the stomach can vary according to the patient's BMI when the water-soluble contrast passes into the remnant stomach. The desired pattern for the remnant stomach is a tubular shape due to the bariatric procedure, and an afferent loop is usually unidentified throughout its course (Fig. 2). Although an anchoring suture is made adjacent to the gastro-jejunostomy for angulation to prevent bile reflux and exclude food from the bypassed segment, the afferent loop can be infrequently opacified. Contrast should not pass the gastro-jejunostomy.
A contrast-enhanced CT scan is performed if postoperative complications are detected on the UGI or are clinically suspected. A CT scan shows the remnant stomach, the efferent loop, the bypassed segments, and their relationship with adjacent structures (Fig. 3). The anastomosis site is typically recognized as a highly attenuated staple line and can be used to find the surgical field.
Hemorrhage and hematoma (Fig. 4) usually occur in the surgical field, including the staple line of the stomach and adjacent to the gastro-jejunostomy site. Hemorrhage and hematoma can also occur at a trocar site or from internal solid organs located in the surgical field, such as the liver or spleen, in up to 3.4% of patients undergoing mini-gastric bypass (11). The origins of hemorrhage include insufficient vascular ligation or hemostasis or injury to solid organs (13). When blood through the surgical drainage or incision site is identified and accompanied by clinical hypotension, intra-abdominal hemorrhage clinically manifests. Hematoma and hemorrhage are typically shown as high density fluid or hemoperitoneum (60-80 Hounsfield units) in the acute stage on a CT scan. Because a hematoma is a low density fluid in the chronic stage, it is not easy to distinguish from ascites or an abscess (14).
Stricture or stenosis (Figs. 5, 6) at the gastro-jejunostomy site is a relatively common complication that can result in postprandial vomiting and pain (15). Causes for a passage disturbance can be anastomotic edema secondary to surgery or over-sewing (16). A transient standing column of contrast agent in the esophagus lasts < 1 minute in a typical case of postoperative edema, and recovery is expected within 2 weeks. Early stricture or severe edema should be considered when contrast remains static in the esophagus for > 1 minute. Persistent distension and air-fluid in the remnant stomach and esophagus on follow-up studies suggests a stricture, and balloon dilation using endoscopy is likely necessary (17).
An abscess (Fig. 7) is usually associated with an intestinal leak or perforation (18, 19). CT is the primary means for diagnosing intestinal leaks and abscesses. CT reveals an intra-abdominal abscess as a collection of fluid with rim enhancement, internal gas formation, and adjacent inflammatory changes.
Anastomotic leaks (Fig. 8) are an uncommon but ominous complication and occur in 0.5-1.9% of patients undergoing the surgery (10, 12, 20, 21). A leak is common at the gastro-jejunostomy site. A postoperative UGI is necessary to identify a leak before patients start a liquid diet. Water-soluble contrast agent directly spills into the extra-luminal space and perigastric soft tissue during a UGI study. Pneumoperitoneum, localized fluid collection in an unexpected site, or an abscess are highly suspicious of leakage on a CT scan, and the presence of oral contrast in the extra-luminal space is obvious evidence of leakage. Although the majority of leaks are detected during the first 7 PODs, some leaks can occur later (22). Immediate surgical exploration should be advocated if a leak is strongly suspected, even with a negative postoperative UGI result.
Wound complications (Fig. 9) are easily detected at the incision site. Subcutaneous fat stranding, mottled air bubbles, or a small volume of fluid in an adjoining trocar site are expected as postoperative findings. A wound infection may result from inflammation or necrosis of wound closure material and form an abscess. A larger volume of fluid and severe fat stranding should be considered an abscess. Wound dehiscence as a consequence of loosening of closure material or an incision hernia at the trocar site can be detected on a CT scan (14).
Marginal ulcers (ulcer at the gastrojejunal anastomosis) are one of the most problematic postoperative complications following gastric bypass surgery. The main factors leading to a marginal ulcer are a large gastric tube and exposure to acid, typically on the jejunal side (23, 24). Marginal ulcers occur in 0.6-8.0% of patients undergoing the surgery (9, 10, 12, 25).
Detecting a marginal ulcer (Fig. 10) on a UGI study or a CT scan is less reliable than endoscopy; therefore, these radiological examinations may produce negative results despite a positive endoscopic result. Although an ulcer is typically treated medically, surgery is required in cases of perforation, hemorrhage, ulcer intractability, or obstruction (24). Edematous wall thickening at the involved site, including perilesional fat stranding, perforation, or abscess may be seen on CT, and a stenosis or deformity can be identified as a result of scarring or fibrosis.
The incidence of cholelithiasis increases by approximately 10-40% in patients with morbid obesity, compared with those of normal weight. The risks for gallbladder (GB) stones and cholecystitis (Fig. 11) increase following the rapid weight loss after bariatric surgery, ultimately leading to cholecystectomy in 20-30% of patients. Thus, many surgeons perform a pre-emptive cholecystectomy concomitantly with bariatric surgery (26). Typical CT findings of acute cholecystitis include GB wall thickening, distension, subserosal edema of the GB, high-attenuation bile, and fluid stranding and collection in an adjacent area (27).
The bypassed segment of a mini-gastric bypass includes the stomach, duodenum, and proximal jejunum. A spontaneous perforation in the bypassed segment (Fig. 12) in a patient who has undergone mini-gastric surgery has not been reported. Macgregor et al. (28) reported a perforation in the bypassed segment after Roux-en-Y gastric bypass in < 0.24% of patients, including the duodenum and stomach. The mechanism of perforation is a complication of peptic ulcer disease or an obstruction of the biliopancreatic limb due to internal herniation (28). Because an endoscopic or fluoroscopic approach is not available for the bypassed segment, a CT scan is necessary to confirm the diagnosis.
Figures and Tables
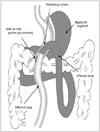 | Fig. 1Illustration of laparoscopic mini-gastric bypass.Mini-gastric tube is mounted parallel from angle of His to 2 cm proximal to pylorus along lesser curvature. Anchoring suture is used for acute angulation, and antecolic side-to-side gastro-jejunostomy is formed 200 cm distal to Treitz ligament. Bypassed segment is denoted by gray zone.
|
 | Fig. 2Normal upper gastrointestinal series on postoperative day 1.
A. No obvious contrast leakage or luminal narrowing is seen at side-to-side gastro-jejunostomy site (arrows). Afferent loop is not typically detected, and faint staple lines (arrowheads) are detected along gastric curvature. B. Image in another patient shows afferent loop (curved arrow) partially and multiple clips (arrows) with faint staple lines to prevent bleeding. E = efferent loop
|
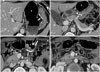 | Fig. 3Computed tomography (CT) scans after mini-gastric bypass surgery.Cranial to caudal CT scans reveal remnant stomach (S) and bypassed segment (B). Gastro-jejunostomy (A) and distal loop (E) are anterior to transverse colon (C). Third duodenum (*) is marker to identify afferent loop. Staples for gastric separation are indicated by white arrows, and side-to-side gastro-jejunostomy is indicated by black arrow.
|
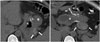 | Fig. 4Formation of hematoma in 31-year-old woman with decreased hemoglobin level.Pre-contrast computed tomography scans reveal high density fluid (arrows) (mean, 58 Hounsfield units) around remnant stomach (S) adjacent to staples without extravasation. Bleeding was spontaneously controlled. B = bypassed segment
|
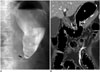 | Fig. 5Anastomotic narrowing in 37-year-old man.
A. Remnant stomach was distended on upper gastrointestinal series due to passage disturbance on postoperative day 1. String-like narrowing at gastro-jejunostomy (arrow) was probably caused by postoperative edema. Follow-up X-ray 4 hours later shows unimpeded passage of contrast agent into large intestine (not shown). B. Follow-up coronal computed tomography scan after 3 months reveals improvement in narrowing of gastro-jejunostomy (arrows) and no evidence of complications. E = efferent loop, S = remnant stomach
|
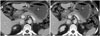 | Fig. 6Anastomotic stenosis in 49-year-old woman with postprandial abdominal pain 6 months postoperatively.Axial computed tomography scans reveal distension of remnant stomach (S) and narrowing of gastro-jejunostomy (arrows), suggesting anastomotic stenosis. B = bypassed segment
|
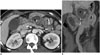 | Fig. 7Formation of abscess in 53-year-old woman with fever on postoperative day 15.Axial (A) and coronal (B) computed tomography scans show rim enhancement and loculated fluid (*) around pancreas with adjacent fat stranding. Percutaneous drainage with catheter produced odorous abscess. B = bypassed segment, S = remnant stomach
|
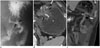 | Fig. 8Anastomotic leak in 46-year-old man with abdominal pain on postoperative day 7.
A. Upper gastrointestinal series on postoperative day 1 shows edematous changes in gastro-jejunostomy (black arrows) without obvious contrast agent leak or passage disturbance. B, C. Axial (B) and coronal (C) computed tomography scans obtained on postoperative day 7 reveal air containing fluid (white arrows) adjacent to anastomosed site (black arrows) and ascites around lesion and left paracolic gutter. A = afferent loop, E = efferent loop, S = stomach
|
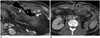 | Fig. 9Wound complications in 46-year-old man who underwent additional Roux-en-Y gastric bypass jejunostomy to treat leak.
A. Computed tomography (CT) scan obtained on postoperative day 37 reveals direct connection (arrow) between remnant stomach and subcutaneous tissue, presenting as gastro-cutaneous fistula. Curved arrow = gastrojejunostomy. B. CT scan obtained on postoperative day 97 reveals wound dehiscence (arrows) and skin thickening. Intra-abdominal abscess (A) and mesenteric fat infiltration with peritoneal thickening are seen with secondary edematous changes in bowel loops. S = remnant stomach
|
 | Fig. 10Marginal ulcer perforation in 65-year-old woman with sudden abdominal pain 1 year after surgery.Axial computed tomography scans reveal focal wall defect (arrows) in efferent loop (E) just below gastro-jejunostomy (A) with pneumoperitoneum (*), associated with edematous wall thickening in remnant stomach (S) as well as efferent loop with mesenteric haziness. B = bypassed segment
|
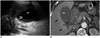 | Fig. 11Acute cholecystitis in 62-year-old woman with upper abdominal pain 1 year after surgery.
A. Abdominal ultrasonography reveals sandy stones and sludge (curved arrow) in distended gall bladder (GB) with wall thickening. B. Computed tomography scan on same day shows distended GB with wall thickening and hyperemia (arrows) of adjacent liver.
|
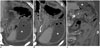 | Fig. 12Spontaneous perforation in bypassed segment of 39-year-old man with generalized abdominal pain 8 months after surgery.Axial (A, B) and coronal (C) computed tomography scans show focal edematous wall thickening and suspicious wall defect (arrows) in upper greater curvature of bypassed gastric segment (B), which was away from staples, with abscess forming (*) around lesion, presumably peptic ulcer with perforation that was surgically proven. S = remnant stomach
|
References
1. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991; 115:956–961.
2. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995; 222:339–350. discussion 350-352.
3. O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006; 144:625–633.
4. Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007; 357:753–761.
5. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003; 238:467–484. discussion 484-485.
6. Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, Chen JC. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI>35 and <35 kg/m2. J Gastrointest Surg. 2008; 12:945–952.
7. Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013; 258:628–636. discussion 636-637.
8. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014; 370:2002–2013.
9. Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005; 242:20–28.
10. Lee WJ, Ser KH, Lee YC, Tsou JJ, Chen SC, Chen JC. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012; 22:1827–1834.
11. Musella M, Susa A, Greco F, De Luca M, Manno E, Di Stefano C, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014; 28:156–163.
12. Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005; 15:1304–1308.
13. Greenfield LJ. Complications of gastric surgery. In : Greenfield LJ, editor. Complications in surgery and trauma. Philadelphia: Lippincott;1990. p. 457–467.
14. Yu J, Turner MA, Cho SR, Fulcher AS, DeMaria EJ, Kellum JM, et al. Normal anatomy and complications after gastric bypass surgery: helical CT findings. Radiology. 2004; 231:753–760.
15. Byrne TK. Complications of surgery for obesity. Surg Clin North Am. 2001; 81:1181–1193. vii-viii.
16. Akkary E, Duffy A, Bell R. Deciphering the sleeve: technique, indications, efficacy, and safety of sleeve gastrectomy. Obes Surg. 2008; 18:1323–1329.
17. Koehler RE, Halverson JD. Radiographic abnormalities after gastric bypass. AJR Am J Roentgenol. 1982; 138:267–270.
18. Blachar A, Federle MP. Gastrointestinal complications of laparoscopic roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. AJR Am J Roentgenol. 2002; 179:1437–1442.
19. Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003; 138:957–961.
20. Carbajo M, García-Caballero M, Toledano M, Osorio D, García-Lanza C, Carmona JA. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005; 15:398–404.
21. Noun R, Skaff J, Riachi E, Daher R, Antoun NA, Nasr M. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg. 2012; 22:697–703.
22. Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005; 15:1252–1256.
23. Jordan JH, Hocking MP, Rout WR, Woodward ER. Marginal ulcer following gastric bypass for morbid obesity. Am Surg. 1991; 57:286–288.
24. Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007; 21:1090–1094.
25. Wang W, Wei PL, Lee YC, Huang MT, Chiu CC, Lee WJ. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005; 15:648–654.
26. Papavramidis S, Deligianidis N, Papavramidis T, Sapalidis K, Katsamakas M, Gamvros O. Laparoscopic cholecystectomy after bariatric surgery. Surg Endosc. 2003; 17:1061–1064.
27. Fidler J, Paulson EK, Layfield L. CT evaluation of acute cholecystitis: findings and usefulness in diagnosis. AJR Am J Roentgenol. 1996; 166:1085–1088.
28. Macgregor AM, Pickens NE, Thoburn EK. Perforated peptic ulcer following gastric bypass for obesity. Am Surg. 1999; 65:222–225.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download