Abstract
Optical imaging techniques use visual and near infrared rays. Despite their considerably poor penetration depth, they are widely used due to their safe and intuitive properties and potential for intraoperative usage. Optical imaging techniques have been actively investigated for clinical imaging of lymph nodes and lymphatic system. This article summarizes a variety of optical tracers and techniques used for lymph node and lymphatic imaging, and reviews their clinical applications. Emerging new optical imaging techniques and their potential are also described.
Optical imaging that uses nonionizing radiation in the visible to near infrared (NIR) wavelengths has several advantages when used for biomedical purposes. For instance, nonionizing light does not harm human health, and optical imaging can provide intuitive visual information. These safe and intuitive properties render optical imaging potentially helpful and practical for intraoperative use.
On the other hand, wavelengths used in optical imaging have shallow penetrating power to view the inside of the entire human body, mainly due to attenuation and scattering. In optical light, NIR in the range of 700-1000 nm in wavelength has relatively high tissue penetration depth of up to one centimeter, in contrast to that of visual ray (less than one millimeter), and produces low autofluorescence from the human body, which leads to a high signal to background ratio (1, 2). Nevertheless, even penetration power of NIR is still insufficient to reveal deep tissues of human body. These properties have hindered clinical usage of optical imaging.
A practical method to overcome the drawbacks of optical imaging is to determine the appropriate clinical indications for which shallow penetration power suffices. Thus, lymph node and lymphatic imaging is assumed as the most appropriate medical application for this technique. Intraoperative imaging might be the best application since dense overlying tissue could be moved aside during the operation. Finally, a combination of optical imaging and other imaging modalities may yield a synergic effect.
For lymphatic imaging, contrast agent is generally needed to be introduced into the lymphatic vessel. Once cannulation is made into the lymphatic vessel, contrast agent can be directly injected and visualize it. However, this method is not preferred because of its technical difficulty and its invasiveness often results in severe complications. Alternatively, an interstitial route to lymphatics can be used. After subcutaneous or intradermal injection, the contrast agents are transported into the lymphatic system. In this method, the particle size of the contrast agent is the most important factor to determine imaging quality. A particle size of approximately 10 nm is preferred because particles < 6 nm quickly diffuse into the surrounding tissues after entering the lymphatic vessel and particles > 100 nm tend to remain in the interstitium. With this understanding, optical tracers have been modified to meet the biosusceptibility and size conditions enabling their use in lymphatic imaging in clinical practice.
Herein, we state review optical tracers and techniques that are used medically and review their clinical applications obtained from a literature search. Other emerging optical imaging techniques and their potential are also described.
In general, substances that absorb light energy change to an unstable excitation state from the ground state and then revert back to the ground state after energy transition. This energy transition includes vibrational energy, heat energy, and light energy (fluorescence). A substance that selectively absorbs light of a certain wavelength is called a chromophore. When it emits fluorescence after absorption of excitation light, it is called a fluorophore. In most cases, fluorescent light has lower energy and a longer wavelength relative to the excitation light, since fluorophores release a certain amount of energy in the form of vibrational relaxation before photoemission.
For fluorescence imaging, a fluorescence-sensor, commonly a fluorescence camera, detects the filtered fluorescence of the typical wavelength emitted by the fluorophore, which reflects the biological process of the body. Some fluorophores can be administered into the body to act as a kind of imaging contrast medium; these fluorophores are called exogenous fluorophores. The body intrinsically emits fluorescence, as many components of the body exhibit weak fluorescence (autofluorescence); these components are called endogenous fluorophores. Since autofluorescence changes in some pathologic conditions, autofluorescence imaging can have biomedical relevance. Since both endogenous and exogenous fluorophores have specific wavelengths at which they absorb light for fluorescence emission and at which they emit fluorescence, it is important to select appropriate excitation wavelengths and filtering conditions to ensure emission of light for good quality fluorescence imaging.
Autofluorescence is natural tissue fluorescence emitted by endogenous fluorophores, such as flavin and collagen (3). These emissions differ based on the tissue type due to biologic features, such as fluorophore concentration (3). Endogenous fluorophores are usually excited by visible light wavelengths from 400-700 nm, with hemoglobin as the main chromophore. Therefore, increased blood volume may also affect tumor autofluorescence. Autofluorescence imaging uses these differences in fluorescent emission intensities between lesions and normal tissue to facilitate lesion detection. Currently, autofluorescence imaging is most commonly used for ocular and gastrointestinal imaging, and is useful for detecting early esophageal squamous cell carcinoma (4, 5). Autofluorescence imaging can be safely applied, since exogenous fluorescent contrast is not administered into the body, which can be potentially toxic. However, the signal to background ratio in autofluorescence imaging is generally low due to subtle autofluorescence differences between normal tissue and lesions; its specificity to identify lesions is insufficient to be used alone for diagnosis. Although video endoscopes improve spatial resolution and color contrast over earlier fiber optic endoscopes (6), further technical advances in autofluorescence imaging are required for it to be used as a stand-alone diagnostic modality in clinical practice (7).
Exogenous fluorophores are preferred over autofluorescence for image contrast in biomedical optical imaging. Exogenous fluorophores are mainly classified into organic and inorganic fluorophores. Organic fluorophores include fluoresceins, rhodamines, and most cyanines. Among the many kinds of fluorophores, fluorescence tracers for biological application are limited to biomolecules that emit NIR wavelengths to minimize autofluorescence and improve signal to background ratios.
In addition to fluorescent properties, potential toxicity must also be considered for biological applications. Currently, the United States Food and Drug Administration (FDA)-approved fluorescent contrasts include only fluorescein and indocyanine green (ICG). Newer organic fluorophores, including sulfonated indocyanine dyes (e.g., Cy 7), sulfonated carbocyanine dyes (e.g., Alexa Fluor 750), and sulfonated rhodamine dye (e.g., Alexa Fluor 633), are synthetic fluorescent dyes with better optical characteristics and are more photostable and brighter than the preexisting original dyes (8). Sulfonation of the molecular surfaces of these organic fluorophores leads to hydrophilicity. However, they are currently not FDA approved. Besides, they must be conjugated to antibodies, peptides, or proteins as optimal lymphatic tracers due to their small size (9, 10, 11). Since the conjugations of fluorophore and macromolecules may have a different toxicity profile from its components, their toxicity must be validated before clinical application. Furthermore, the optimal fluorescent signals are still insufficient to provide adequate image quality as compared to inorganic fluorophores, although their fluorescence is more intense than fluorescein or ICG.
Fluorescein is the most common example of a fluorescent contrast, which was approved by the FDA in 1976. In the literature, about 5% of patients experience adverse reactions to intravenous fluorescein, and the most common symptom is nausea and vomiting (12). Fluorescein is a dark orange-to-red powder with an absorption peak of 494 nm and an emission peak of 521 nm. Fluorescein has a higher quantum yield compared to ICG; however, self-absorption of fluorescence by the surrounding tissue with fluorescein is more significant than ICG in vivo due to the relatively low absorption and emission peaks of the former.
Indocyanine green is the most commonly used NIR fluorescent contrast medium for lymphatic imaging in clinical practice. ICG was introduced to measure cardiac output and was granted FDA approval in 1959 (13). During its more than 50 years of use in various clinical settings, adverse reactions to ICG have been rarely reported (14). In general, ICG is regarded as safe; however, caution should be exercised in case of patients with renal insufficiencies (15). Although ICG appears dark green under natural light, it appears more fluorescent than green once injected into the human body in amounts < 20 mg (Fig. 1) (16). Its absorption peak is approximately 780 nm and its emission peak is approximately 830 nm in a dilute aqueous solution. Although ICG has large overlapping absorption and emission spectra, it can still be easily detected by NIR fluorescence imaging systems. Motomura et al. (17) first reported the potential of ICG as a tracer for sentinel node mapping. However, this technique is merely a substitution for blue dye without a fluorescent detecting system. In the aforementioned article, the success rate for detecting sentinel nodes guided by ICG was 74% of 172 breast cancer patients. Kitai et al. (18) reported a new sentinel lymph node mapping technique guided by ICG fluorescence using a hand-held fluorescence detection device in breast cancer patients with a detection rate of 94%. This was the first reported use of ICG as a NIR dye. Detailed clinical uses will be reviewed in the following section.
A quantum dot (QD) is a nano-sized crystal composed of semiconductor materials. When QDs absorb enough light energy to cause an electron to leave the valence band and to enter the conduction band, an electron-hole pair is produced. During the process of the reunion between the electron and the hole, light is emitted (19, 20). The emission wavelength is closely associated with both the composition and size of the QD. Consequently, the wavelength is dependent on the particle size when the material that makes up QDs is the same. Therefore, by changing the size of QDs, various emission wavelengths can be simultaneously produced by a single excitation light, which enables multiplexed biological imaging. In vivo multicolor lymphatic imaging technique with quantum dots has been successfully demonstrated in animal models (21, 22).
In addition, a number of advantages over conventional fluorophores make QD an attractive optical imaging material. QDs have a wider excitation range and a sharp and nearly symmetrical emission peak (19). Therefore, autofluorescence and background scattering can be minimized by excitation wavelengths far from the emission peak and still within the absorption spectra. Furthermore, QDs have higher quantum yields and greater penetration depth. Lastly, QDs are photostable for longer and are resistant to photobleaching due to their inorganic composition (23, 24). This enables temporal discrimination of a true signal from autofluorescence and scattering. Overall, QDs provide good optical image quality with higher resolution greater sensitivity.
While QDs have desirable qualities for optical imaging, concerns regarding toxicity need to be addressed. Although data about toxicity of QDs is not consistent, QD toxicity has been described (25). Uncoated cadmium, which is commonly used for core semiconductor of QDs, is cytotoxic. Therefore, the metalloid core is capsulated by inorganic shell to stabilize the core and reduced toxicity. Still, possible leakage of the toxic core heavy metal out of shell is the major concern since core-shell complex may become labile under oxidative and photolytic conditions. With this concern, attention has been paid to cadmium-free QDs. Many researchers are studying to develop novel cadmium-free QDs with good performance comparable to currently used cadmium containing QDs but with low toxicity. Nevertheless, their present biomedical use is limited to the preclinical stage.
Unlike optical imaging that has intrinsic limited penetration depth, clinically-approved imaging modalities, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), computed tomography, and magnetic resonance imaging (MRI), have sufficient penetrating power and have irreplaceable individual clinical utility. Multimodal imaging, which combines optical techniques with clinical techniques, can achieve synergistic effects.
The recent development of nanotechnology enables the production of new agents combining both a PET tracer and fluorescent tracer, allowing the convenient use of the combined system with single injection (26, 27). In addition to combining with PET, fluorescence and gamma tracers can also be conjugated for clinical imaging and intraoperative guidance (16, 28). Radionuclide provides deep tissue imaging of the whole body and optical imaging enables longitudinal study even after radionuclide decay. Moreover, optical and PET or SPECT imaging can be cross-validated with dual function probes, since replacing isotopes with fluorescent markers may affect the biodistribution of a tracer (28).
MR lymphography can provide excellent anatomical information of deep lymphatic channels and lymph nodes using gadolinium or iron oxide-based contrast. While MRI is a good modality for preoperative planning, NIR imaging is a good modality as a real-time intraoperative guidance. Studies introducing NIR/MR dual probes for lymphatic imaging have been reported (29, 30). With these probes, both NIR and MR imaging can be obtained by single injection.
In lymphatic imaging, active targeting tracers can specifically bind to a target molecule, such as lymphatic endothelium or metastatic lymph nodes, whereas a fluorophore just drains into lymphatic vessels without any specific binding affinity to the lymphatic tissue when it is subcutaneously administered. The common strategy of generating an active fluorescent targeting tracer is direct conjugation of a fluorophore with specific ligands to recognize target molecules. Another recent strategy is through chemical reaction, such as enzymatic cleavage to switch from a less fluorescent form into a highly fluorescent form when approaching target molecules.
Lymph vascular endothelial receptor (LYVE-1) is a specific marker of lymphatic endothelium. Sharma et al. (31) introduced a novel optical targeting tracer for LYVE-1 combining hyaluronic acid and IR 783 dye. Later, McElroy et al. (11) also demonstrated that the fluorescent signal of the LYVE-1 antibody was durable enough to image mouse lymphatics.
There have also been attempts to generate molecules that can actively target metastatic lymph nodes. Sampath et al. (32) reported a dual-labeled antibody-based probe specific for HER2, (111In-DTPA)n-trastuzumab-(IRDye800)m, for detecting lymph nodes in a human breast cancer xenograft mouse model. Using the same dual-labeled antibody-based probe strategy, but with copper instead of indium as the label, (64Cu-DOTA)n-trastuzumab-(IRDye800)m was introduced for HER2 positive breast cancer cell imaging (33). Dual-labeled probes for epithelial cell adhesion molecule (EpCAM), (64Cu-DOTA)n-anti-EpCAM-(IRDye800)m, were demonstrated to detect tumor and metastatic lymph nodes (34). In the aforementioned articles, a combination of optical and PET imaging successfully visualized primary tumors and metastatic lymph nodes.
Although active targeting has been reported to have some advantages over traditional optical imaging techniques, such as higher lymph node uptake and lower nonspecific background uptake, thereby leading to increased target to background ratio and better spatial resolution (32), few human studies have been attempted. To date, active targeting tracers have less optimal specificity than expected and medical usage is not yet approved due to structural complexity issues and potential toxicity.
Photoacoustic imaging is a hybrid imaging method using exciting nonionizing light (pulsed laser) and emitted ultrasound signals from endogenous or exogenous chromophores. When laser pulses are delivered into tissues, some of them are absorbed into endogenous or exogenous chromophores and are converted to heat. The heat leads to pulsatile thermal expansion and subsequent relaxation of the tissue with a negligible temperature rise, which does not result in physical damage. This serial process generates wideband acoustic emissions with much greater penetration depth than that of fluorescence emission, which overcomes the depth limitation of fluorescent light (9, 35, 36). An acoustic detector receives the propagated ultrasound at the surface, and the image is reconstructed by measuring propagation time, similar to typical ultrasound imaging.
In the body, biologic components, such as hemoglobin, melanin, or water, act as endogenous chromophores. Photoacoustic signals from the body are highly related to changes in these specific components. Therefore, the intensity of the photoacoustic signal can reflect the physiological nature of the tissue. Since blood has sufficient endogenous chromophores, it provides high contrast for photoacoustic imaging to visualize blood vessels. Common uses of photoacoustic imaging are for monitoring tumor angiogenesis, blood oxygenation in tissues, and detecting skin melanomas.
Exogenous chromophores can be utilized to further increase image contrast and can be used for sentinel lymph imaging by subcutaneous injection. These contrast agents could be methylene blue or nanostructures that absorb NIR light. Photoacoustic imaging can easily be applied in human studies as methylene blue, a FDA-approved vital blue dye originally used as a lymphatic tracer, can be used as an exogenous chromophore and a clinical ultrasound apparatus can be used without major modification (37). This approach for detecting sentinel lymph nodes is currently being studied in clinical trials in breast cancer patients (38). Nanostructures, such as carbon nanotube, gold shell, or gold nanocages, are under study but now are limited to preclinical use. Since photoacoustic imaging methods can be used to visualize deep tissues, it is expected that it will be used for diverse clinical applications in addition to lymph or lymphatic imaging.
Despite the high potential of this methodology, some technical problems remain to be solved. For instance, the bulky size, complexity, and price of the pulsed laser apparatus are the main hurdles to widespread clinical application.
Diffusion optical imaging is also an extension of the NIR fluorescence imaging method, but its uniqueness lies in its ability to detect the amount of absorption and scattering of the NIR light as it travels through tissue. Detection of optical signals is technically challenging due to the large amount of absorption and scattering when light travels through tissues at optical wavelengths. There have been many efforts to minimize the scattering effect and to improve the spatial resolution of optical imaging. NIR has been the focus due to its high penetration ability; however, NIR light causes a lot of scatters which prevent acquiring good images through tissues more than 5 mm thick. Conversely, some researchers accept scattering as an inevitable factor of optical imaging and instead focus on large volume and lower resolution imaging methods. The amount of absorption and scattering depends on differences in the chemical and physiologic composition of the tissue. Therefore, diffusion optical imaging is functional imaging for revealing tissue characteristics with respect to tissue components, providing delicate anatomical information.
The transillumination method for hydrocephalus is considered to be the first clinical application of diffusion optical imaging. Recently, diffusion optical imaging has been used to image brain function and the breast (39). However, this technique is assumed to be inappropriate for lymph nodes and lymphatic images.
A summary of the aforementioned three optical imaging techniques is provided in Table 1.
The concept of a sentinel node was put forward around the end of the 20th century (40). Morton et al. (41) demonstrated lymphatic mapping in melanoma with colloidal gold in 1977, and later, in 1992, with blue dye. In breast cancer as well as melanoma, sentinel nodes are visualized with blue dye (42) and with radiolabeled colloids (43). Since the introduction of sentinel node mapping with vital dye, sentinel node biopsy has been widely adopted as an alternative to invasive radical lymphadenectomy. Now, sentinel node biopsy is the standard procedure for early-stage breast cancer and melanoma with clinically-negative lymph nodes (44, 45).
Although either blue dye or radionuclides are typically used for intraoperative sentinel node mapping, better detection rates have been reported when combining blue dye with radionuclides (46). Blue dye can be used as a good intraoperative visual guide for lymphatic drainage, and radionuclides are good for preoperative planning. While blue dye can offer real-time visual guidance, blue dye alone essentially needs more skin excision to remove the sentinel node since the blue color of the sentinel node cannot be easily seen through the skin. While radionuclides have great penetration ability, radionuclides alone require a certain amount of time for the surgeon to adjust to the system because radionuclide cannot be used for real-time visual guidance. Therefore, NIR fluorescence lymphangiography has been studied for more than a decade to overcome these issues (47).
Near infrared fluorescence lymphography has some advantages over conventional modalities, as it provides real-time visual guidance with relatively good penetration. Currently, various sizes of fluorescence imaging systems have been developed for clinical application from a hand-held system to a room-based system (Fig. 2). Since ICG is the only clinical lymphatic tracer used that is currently approved by the FDA and by the European Medicines Agency, many researchers have tried to apply ICG for lymph node mapping in a variety of cancers, including breast cancer, melanoma, vulvar cancer, and gastric cancer (48, 49, 50, 51).
Breast cancer is the most researched cancer for ICG-guided sentinel lymph node biopsy (16, 30, 48, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62). In general, ICG showed good performance for sentinel node detection (Table 2, Fig. 2). Although the detection rate of sentinel lymph nodes by ICG is 100% in most cases, transcutaneous visualization is variable from 31% to 94%. In the most recent study conducted by Verbeek et al. (48), 100% of sentinel nodes were detected with ICG fluorescence, while 88% of sentinel nodes were detected with radioscintigraphy. However, radioscintigraphy was required for initial localization in two cases whose sentinel nodes were missed by ICG fluorescence alone. These results suggest that transcutaneous visualization of axillary lymph nodes by ICG is still limited due to the penetration ability of NIR compared to radionuclides and it is difficult to find some sentinel lymph nodes, especially the deep-seated axillary lymph node using ICG fluorescence signal only. Overall, it appears that ICG is a superior alternative for sentinel lymph node mapping to blue dye, although radioscintigraphy still has a role for initial localization of deep-seated sentinel lymph nodes. Further studies, including investigating multimodal imaging techniques, for improving localization and detection are needed to determine the gold standard among those modalities.
With respect to intraoperative usage, human eyes are insensitive to NIR light from ICG, which could be a double-edged sword for its clinical application. This property of NIR tracers might be an advantage over blue dye as surgical fields are not obscured when NIR fluorescent tracer is used. Conversely, NIR tracer always requires an additional device/equipment to detect the fluorescent signal.
Lymphedema can be divided into two categories based on pathology. Primary lymphedema is a congenital form due to a genetic defect, while secondary lymphedema is an acquired form due to lymphatic vessel damage. As the number of cancer patients increases and their survival improves, secondary lymphedema as a surgical complication has become widespread. In most cases, conservative treatment is applied, and microsurgery, such as lymphatic-venous anastomosis, has proven to be successful for treating early peripheral lymphedema that is not sufficiently responsive to conservative treatment (63). Therefore, detection of early stage lymphedema is important to identify those in whom surgical procedures would be helpful and follow-up is needed to evaluate surgical outcome.
There have been many efforts to find alternatives for indirect visualization of lymphatic vessels, since direct lymphangiography is invasive and often results in severe complications. Although lymphoscintigraphy is the most commonly used method to visualize lymphatic circulation, recent studies have demonstrated the value of ICG lymphography to evaluate lymphedema. Mihara et al. (64, 65) performed comparative studies between ICG lymphography and lymphoscintigraphy. The authors reported that ICG lymphography had better sensitivity than lymphoscintigraphy, while the two modalities showed equally good specificity in mild upper limb lymphedema (64). In another study, better sensitivity and specificity of ICG lymphography in secondary lymphedema of the lower limbs were realized, especially for diagnosis of early lymphedema (65). With these results and the advantages of ICG lymphography over lymphoscintigraphy, such as real-time intraoperative visualization and no radiation exposure, ICG lymphography seems to be a suitable alternative for surgical planning and post-treatment follow-up.
New advances in optical probes hold great promise for optical imaging. Active fluorescent dyes that target specific ligands such as LYVE-1 and HER2 are promising techniques of the future. On the other hand, multimodal probes, such as combining ICG and a radionuclide, might be utilized in clinical practice in the near future as a lymphatic imaging tracer.
Recent technological developments have generated new optical imaging concepts, such as photoacoustic imaging and diffusion optical imaging, to improve tissue penetration. The potential of these new techniques has been validated in humans in recent years. Further development and testing are needed before applying these techniques in clinical practice.
Recently, optical imaging for the lymphatic system and lymph nodes is being used in specific clinical areas. Among the various optical imaging techniques and tracers, NIR fluorescence imaging using ICG is the only de facto clinically adopted technique by virtue of its clinical availability and technical ease. However, ICG fluorescence imaging is insufficient for solving all existing clinical needs, such as detecting deep-seated lymph nodes. Therefore, novel optical techniques that are cost-effective, technically convenient to use, and able to assess and image deeper tissues, are required for wider applications in the near future.
Figures and Tables
Fig. 1
Infrared fluorescence of indocyanine green (ICG) is more intense in dilute condition with weaker green color than in concentrated condition.
A. Commercial ICG green kit. B. Photos of 0.25% ICG aqueous solution (right vial) and 0.002% ICG aqueous solution (left vial). 0.25% ICG solution is dark green in color, while 0.002% ICG aqueous solution is faint green. Near infrared (NIR) fluorescence images were obtained using fluorescence imager. C. NIR image with excitation light on. 0.002% ICG aqueous solution (left vial) shows intense fluorescence with minimal background.

Fig. 2
Various types of fluorescence imagers can be applied to visualize tissues stained with indocyanine green (ICG) in clinical applications.
A. They range from small, simple, hand-held type to large room-based type. B. ICG solution is injected subcutaneously into periareolar area before operation. C. Lymphatic flows (arrowheads) can be assessed using near infrared fluorescence imager over intact breast skin. D. During operation, intense fluorescence is identified in sentinel lymph node (arrows).
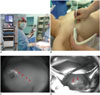
Table 1
Summary of Characteristics of Major Optical Imaging Techniques

| Modality | Fluorescence Imaging | Photoacoustic Imaging | Diffuse Optical Imaging |
|---|---|---|---|
| Tracers available in clinics | ICG, fluorescein | Blue dye, ICG | ICG |
| Excitation range | Visual and near infrared ray (about 400–1000 nm) | Visual and near infrared ray (about 500–1064 nm) (66) | Near infrared ray (700–1000 nm) |
| Emission range | Visual and near infrared ray (about 400–1000 nm) | Acoustic wave | Near infrared ray (700–1000 nm) |
| Penetration depth (mm) | Practically less than 10 mm | -40 mm (50–60 mm with contrast) | Generally -40 mm |
| Spatial resolution | High | Low (submillimeter) | Lowest* (5 mm to 10 mm) |
| Signal to background ratio | High with contrast | Low | Low |
| Real-time intraoperative imaging | Feasible | Limited | Not feasible |
| Common clinical application | Intraoperative imaging | US-guided SLN needle biopsy | Breast cancer functional imaging |
| SLN mapping | |||
| Lymphedema |
Table 2
Representative Clinical Studies on Use of Fluorescence Lymphography with ICG for Sentinel Lymph Node Detection

| Author, Year | Combined Tracer | No. of Study Patients | Average No. of SLN | Detection Rate per Patients, % (n) | Transcutaneous Lymphatic Visualization, % (n) | |
|---|---|---|---|---|---|---|
| Hirche et al. (52), 2010 | - | 43 | 2.0 | 98 (42/43) | 88 (38/43) | |
| Hojo et al. (53), 2010 | BD or RT | 141 | 3.8 | 99 (140/141) | N/A | |
| Tagaya et al. (54), 2010 | BD | 150 | 3.7 | 99 (148/150) | 75* (112/150) | |
| Tagaya et al. (67), 2011 | BD | 50 | 3.7 | 100 (50/50) | 64* (32/50) | |
| Aoyama et al. (55), 2011 | - | 312 | 3.4 | 100 (312/312) | 98 (305/312) | |
| Abe et al. (30), 2011 | BD | 128 | 3.1 | 100 (218/218) | 100 (218/218) | |
| Hirche et al. (56), 2012 | - | 47 | 2.0 | 98 (46/47) | 98 (46/47) | |
| Takeuchi et al. (57), 2012 | BD | 145 | 3.6 | 99 (144/145) | 100 (79/79) | |
| Hirano et al. (58), 2012 | BD | 108 | 2.2 | 99 (107/108) | N/A | |
| Kitai and Kawashima (59), 2012 | - | 50 | 3.6 | 100 (50/50) | 94† (47/50) | |
| Wishart et al. (60), 2012 | BD, RT | 99 | 2.0 | 100 (99/99) | 100 (99/99) | |
| Sugie et al. (61), 2013 | BD | 99 | 3.4 | 99 (98/99) | N/A | |
| Jung et al. (16), 2014 | RT, BD | 43 | 3.4 | 100 (43/43) | 91 (39/43) | |
| Ballardini et al. (62), 2013 | RT | 134 | 1.8 | 100 (245/246)* | N/A | |
| Verbeek et al. (48), 2014 | RT | 95 | 1.9 | 100 (177/177)* | 31† (29/95) | |
References
1. van der Vorst JR, Schaafsma BE, Hutteman M, Verbeek FP, Liefers GJ, Hartgrink HH, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 2013; 119:3411–3418.
2. Vellekoop IM, Aegerter CM. Focusing light through living tissue. Proc SPIE. 2010; 7554:755430.
3. ASGE Technology Committee. Song LM, Banerjee S, Desilets D, Diehl DL, Farraye FA, et al. Autofluorescence imaging. Gastrointest Endosc. 2011; 73:647–650.
4. Yoshida Y, Goda K, Tajiri H, Urashima M, Yoshimura N, Kato T. Assessment of novel endoscopic techniques for visualizing superficial esophageal squamous cell carcinoma: autofluorescence and narrow-band imaging. Dis Esophagus. 2009; 22:439–446.
5. Suzuki H, Saito Y, Ikehara H, Oda I. Evaluation of visualization of squamous cell carcinoma of esophagus and pharynx using an autofluorescence imaging videoendoscope system. J Gastroenterol Hepatol. 2009; 24:1834–1839.
6. Uedo N, Iishi H, Tatsuta M, Yamada T, Ogiyama H, Imanaka K, et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005; 62:521–528.
7. Falk GW. Autofluorescence endoscopy. Gastrointest Endosc Clin N Am. 2009; 19:209–220.
8. Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, et al. Quantitative comparison of long-wavelength Alexa Fluor dyes to Cy dyes: fluorescence of the dyes and their bioconjugates. J Histochem Cytochem. 2003; 51:1699–1712.
9. Zhang F, Niu G, Lu G, Chen X. Preclinical lymphatic imaging. Mol Imaging Biol. 2011; 13:599–612.
10. Hama Y, Koyama Y, Urano Y, Choyke PL, Kobayashi H. Two-color lymphatic mapping using Ig-conjugated near infrared optical probes. J Invest Dermatol. 2007; 127:2351–2356.
11. McElroy M, Hayashi K, Garmy-Susini B, Kaushal S, Varner JA, Moossa AR, et al. Fluorescent LYVE-1 antibody to image dynamically lymphatic trafficking of cancer cells in vivo. J Surg Res. 2009; 151:68–73.
12. Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging. 2009; 8:341–354.
13. Fox IJ, Brooker LG, Heseltine DW, Essex HE, Wood EH. A tricarbocyanine dye for continuous recording of dilution curves in whole blood independent of variations in blood oxygen saturation. Proc Staff Meet Mayo Clin. 1957; 32:478–484.
14. Bjerregaard J, Pandia MP, Jaffe RA. Occurrence of severe hypotension after indocyanine green injection during the intraoperative period. A A Case Rep. 2013; 1:26–30.
15. Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn. 1989; 17:231–233.
16. Jung SY, Kim SK, Kim SW, Kwon Y, Lee ES, Kang HS, et al. Comparison of sentinel lymph node biopsy guided by the multimodal method of indocyanine green fluorescence, radioisotope, and blue dye versus the radioisotope method in breast cancer: a randomized controlled trial. Ann Surg Oncol. 2014; 21:1254–1259.
17. Motomura K, Inaji H, Komoike Y, Kasugai T, Noguchi S, Koyama H. Sentinel node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol. 1999; 29:604–607.
18. Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005; 12:211–215.
19. Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. 2002; 13:40–46.
20. Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005; 307:538–544.
21. Hama Y, Koyama Y, Urano Y, Choyke PL, Kobayashi H. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Cancer Res Treat. 2007; 103:23–28.
22. Kosaka N, Ogawa M, Sato N, Choyke PL, Kobayashi H. In vivo real-time, multicolor, quantum dot lymphatic imaging. J Invest Dermatol. 2009; 129:2818–2822.
23. Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005; 7:55–76.
24. Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005; 16:63–72.
25. Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006; 114:165–172.
26. Nahrendorf M, Keliher E, Marinelli B, Waterman P, Feruglio PF, Fexon L, et al. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci U S A. 2010; 107:7910–7915.
27. Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007; 48:1862–1870.
28. Culver J, Akers W, Achilefu S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J Nucl Med. 2008; 49:169–172.
29. Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, et al. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007; 25:866–871.
30. Abe H, Mori T, Umeda T, Tanaka M, Kawai Y, Shimizu T, et al. Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today. 2011; 41:197–202.
31. Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007; 292:H3109–H3118.
32. Sampath L, Wang W, Sevick-Muraca EM. Near infrared fluorescent optical imaging for nodal staging. J Biomed Opt. 2008; 13:041312.
33. Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. Detection of Cancer Metastases with a Dual-labeled Near-Infrared/Positron Emission Tomography Imaging Agent. Transl Oncol. 2010; 3:307–317.
34. Hall MA, Kwon S, Robinson H, Lachance PA, Azhdarinia A, Ranganathan R, et al. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate. 2012; 72:129–146.
35. Song KH, Stoica G, Wang LV. In vivo three-dimensional photoacoustic tomography of a whole mouse head. Opt Lett. 2006; 31:2453–2455.
36. Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol. 2003; 21:803–806.
37. Song KH, Stein EW, Margenthaler JA, Wang LV. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J Biomed Opt. 2008; 13:054033.
38. Erpelding TN, Garcia-Uribe A, Krumholz A, Ke H, Maslov K, Appleton C, et al. A dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection: preliminary clinical results. Proc SPIE. 2014; 8943:894359.
39. Corlu A, Choe R, Durduran T, Rosen MA, Schweiger M, Arridge SR, et al. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt Express. 2007; 15:6696–6716.
40. Jain R, Dandekar P, Patravale V. Diagnostic nanocarriers for sentinel lymph node imaging. J Control Release. 2009; 138:90–102.
41. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992; 127:392–399.
42. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994; 220:391–398. discussion 398-401.
43. Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993; 2:335–339. discussion 340.
44. Sondak VK, Wong SL, Gershenwald JE, Thompson JF. Evidence-based clinical practice guidelines on the use of sentinel lymph node biopsy in melanoma. Am Soc Clin Oncol Educ Book. 2013; http://dx.doi.org/10.1200/EdBook_AM.2013.33.e320.
45. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703–7720.
46. Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010; 17:1854–1861.
47. Xiong L, Gazyakan E, Yang W, Engel H, Hünerbein M, Kneser U, et al. Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol. 2014; 40:843–849.
48. Verbeek FP, Troyan SL, Mieog JS, Liefers GJ, Moffitt LA, Rosenberg M, et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat. 2014; 143:333–342.
49. Jain V, Phillips BT, Conkling N, Pameijer C. Sentinel lymph node detection using laser-assisted indocyanine green dye lymphangiography in patients with melanoma. Int J Surg Oncol. 2013; 2013:904214.
50. Crane LM, Themelis G, Arts HJ, Buddingh KT, Brouwers AH, Ntziachristos V, et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: first clinical results. Gynecol Oncol. 2011; 120:291–295.
51. Miyashiro I, Hiratsuka M, Kishi K, Takachi K, Yano M, Takenaka A, et al. Intraoperative diagnosis using sentinel node biopsy with indocyanine green dye in gastric cancer surgery: an institutional trial by experienced surgeons. Ann Surg Oncol. 2013; 20:542–546.
52. Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010; 121:373–378.
53. Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010; 19:210–213.
54. Tagaya N, Nakagawa A, Abe A, Iwasaki Y, Kubota K. Non-invasive identification of sentinel lymph nodes using indocyanine green fluorescence imaging in patients with breast cancer. Open Surg Oncol J. 2010; 2:71–74.
55. Aoyama K, Kamio T, Ohchi T, Nishizawa M, Kameoka S. Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J Surg Oncol. 2011; 9:157.
56. Hirche C, Mohr Z, Kneif S, Murawa D, Hünerbein M. High rate of solitary sentinel node metastases identification by fluorescence-guided lymphatic imaging in breast cancer. J Surg Oncol. 2012; 105:162–166.
57. Takeuchi M, Sugie T, Abdelazeem K, Kato H, Shinkura N, Takada M, et al. Lymphatic mapping with fluorescence navigation using indocyanine green and axillary surgery in patients with primary breast cancer. Breast J. 2012; 18:535–541.
58. Hirano A, Kamimura M, Ogura K, Kim N, Hattori A, Setoguchi Y, et al. A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann Surg Oncol. 2012; 19:4112–4116.
59. Kitai T, Kawashima M. Transcutaneous detection and direct approach to the sentinel node using axillary compression technique in ICG fluorescence-navigated sentinel node biopsy for breast cancer. Breast Cancer. 2012; 19:343–348.
60. Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012; 38:651–656.
61. Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H, et al. Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol. 2013; 20:2213–2218.
62. Ballardini B, Santoro L, Sangalli C, Gentilini O, Renne G, Lissidini G, et al. The indocyanine green method is equivalent to the 99mTc-labeled radiotracer method for identifying the sentinel node in breast cancer: a concordance and validation study. Eur J Surg Oncol. 2013; 39:1332–1336.
63. Campisi C, Bellini C, Campisi C, Accogli S, Bonioli E, Boccardo F. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010; 30:256–260.
64. Mihara M, Hara H, Araki J, Kikuchi K, Narushima M, Yamamoto T, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One. 2012; 7:e38182.
65. Mihara M, Hara H, Narushima M, Todokoro T, Iida T, Ohtsu H, et al. Indocyanine green lymphography is superior to lymphoscintigraphy in imaging diagnosis of secondary lymphedema of the lower limbs. J Vasc Surg Venous Lymphat Disord. 2013; 1:194–201.
66. Li C, Wang LV. Photoacoustic tomography and sensing in biomedicine. Phys Med Biol. 2009; 54:R59–R97.
67. Tagaya N, Aoyagi H, Nakagawa A, Abe A, Iwasaki Y, Tachibana M, et al. A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg. 2011; 35:154–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download