Abstract
Objective
The purpose of this study was to retrospectively evaluate the diagnostic accuracy and complications of CT-guided core needle biopsy (CT-guided CNB) of pleural lesion and the possible effects of influencing factors.
Materials and Methods
From September 2007 to June 2013, 88 consecutive patients (60 men and 28 women; mean [± standard deviation] age, 51.1 ± 14.4 years; range, 19-78 years) underwent CT-guided CNB, which was performed by two experienced chest radiologists in our medical center. Out of 88 cases, 56 (63%) were diagnosed as malignant, 28 (31%) as benign and 4 (5%) as indeterminate for CNB of pleural lesions. The final diagnosis was confirmed by either histopathological diagnosis or clinical follow-up. The diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and complication rates were statistically evaluated. Influencing factors (patient age, sex, lesion size, pleural-puncture angle, patient position, pleural effusion, and number of pleural punctures) were assessed for their effect on accuracy of CT-guided CNB using univariate and subsequent multivariate analysis.
Results
Diagnostic accuracy, sensitivity, specificity, PPV, and NPV were 89.2%, 86.1%, 100%, 100%, and 67.8%, respectively. The influencing factors had no significant effect in altering diagnostic accuracy. As far as complications were concerned, occurrence of pneumothorax was observed in 14 (16%) out of 88 patients. Multivariate analysis revealed lesion size/pleural thickening as a significant risk factor (odds ratio [OR]: 8.744, p = 0.005) for occurrence of pneumothorax. Moreover, presence of pleural effusion was noted as a significant protective factor (OR: 0.171, p = 0.037) for pneumothorax.
Both neoplastic (primary and metastatic) and nonneoplastic pleural disease can exhibit similar clinical, radiographic and gross features including pleuritic pain, pleural-based masses or pleural thickening and pleural effusion. Accurate differential diagnosis between malignant and benign pleural disease is crucial because of the associated differences in the management and prognosis of respective lesions (1).
The role of ultrasonography (US) in the chest region has traditionally been limited when compared with computed tomography (CT) (2). Consequently, US has been employed for evaluation of pleural effusion, pneumothorax and pulmonary fibrosis (3, 4). Apart from serving as a diagnostic procedure, it also serves as guidance for thoracentesis and chest tube placement (5). Real-time multiplanar monitoring of the procedure along patient's bedside without the use of ionizing radiation is one of the major advantages of US. Thoracoscopy, either medical (pleuroscopy) or surgical (video-assisted thoracoscopic surgery, VATS) has a diagnostic yield of 91-95% for pleural malignancy, and up to 100% for pleural tuberculosis (TB) (6, 7). However, access to thoracoscopy is limited in many healthcare systems due to requirement of significant resources and expertise, including appropriate anesthetic support (8). CT, due to technical advancement, has rapidly become the guidance modality of choice for performing biopsy of pleural lesion; moreover, CT-guided biopsy offers a readily accessible, minimally invasive, rapid and low-cost alternative approach when compared to thoracoscopy to obtain sample tissues (8).
Lung biopsy performed under CT guidance is an established and well-described diagnostic technique. There exist several published guidelines regarding the best practice of this procedure. However, in comparison to CT-guided lung biopsy, fewer reports are available about the risk factors of complications of CT-guided pleural biopsies. Therefore, the purpose of this study was to evaluate diagnostic accuracy and complications of CT-guided core needle biopsy (CT-guided CNB) of pleural lesions and to determine the effect of possible influencing factors.
This retrospective study was approved by the Institutional Review Board and by the ethics commission. The acquisition of informed consent from patients was waived, as the study retrospectively utilized data available in electronic medical records.
From September 2007 to June 2013, a cohort of 88 consecutive CT-guided CNB for pleural lesions in 88 patients (60 men and 28 women; mean [± standard deviation] age, 51.1 ± 14.4 years; range, 19-78 years) was subjected to analyze factors influencing accuracy and complications in patients who underwent CT-guided CNB of pleural lesions at our medical center. The mean interval from diagnostic CT scan to the biopsy procedure was 14.5 days (range: 0-28 days).
Inclusion criteria for the trial were: 1) pleural or peripherally located lung lesions with at least a small degree of contact with the pleura; 2) pleural lesions/pleural thickening > 5 mm in maximum diameter; 3) unilateral pleural effusion with clinical suspicion of malignant pleural disease; 4) at least one negative pleural fluid cytological examination for malignant cells; 5) patient had normal platelet counts, activated partial prothrombin time within 3 days of undergoing needle biopsy; and 6) patient could tolerate a recumbent position. Our exclusion criteria were: 1) pleural lesions/pleural thickening < 5 mm in maximum diameter; 2) centrally located small pleural nodule (< 5 mm) with pleural effusion; 3) transudative pleural effusion (pleural fluid protein < 35 g/dL) associated with heart failure or hypoalbuminemia, such that the clinical prior probability of a hydrostatic effusion was high; 4) pleural fluid cytological examination showing definite malignant cells; 5) any bleeding diathesis sufficient to make pleural biopsy unusually hazardous; and 6) patients who could not follow verbal or visual instruction, and patients or family members who could not accept procedure-related risks.
The patients fasted for at least 4 hours prior to the procedure. The procedure was performed with the patient in a prone, supine or lateral decubitus position depending upon the location of the lesion. Electrocardiography, percutaneous oxygen saturation and blood pressure were monitored constantly during the procedure. The procedure was performed under conscious sedation. Most of the patients had contrast-enhanced chest CT scans available for review before the biopsy procedures. Prior to the procedure, diagnostic CT and new localization CT scan images were carefully reviewed to determine the best target and the most appropriate route for successful and safe tissue sampling. A skin marker (a radiopaque thin stick or grid) was placed on the body surface corresponding to the target lesion. All the patients underwent selected 3 mm sections through the area of interest using a single-section spiral CT machine (Xvision, Toshiba, Tochigi, Japan). Images through the region of interest were viewed under a combination of lung and soft-tissue window settings. To confirm the optimal puncture route, the position of the patient or arm was adjusted and additional CT imaging was performed, if necessary.
At the site of puncture, approximately 3 mL of 1% lidocaine was injected subcutaneously as local anesthesia. Two experienced operators performed CT-guided biopsy procedures with a 19-G needle (TruGuide, Bard, AZ, USA) and image guidance. After needle insertion, a CT scan was done to confirm the position of the needle after which the stylet was removed and core biopsy was performed with the matching 20-G cutting needle (Magnum Needles, Bard, AZ, USA) and a biopsy gun (Magnum, Bard, AZ, USA) (Figs. 1, 2). The operator visually checked adequacy of the specimen. If the sample amount was judged as insufficient, a new inner needle was inserted through the remaining outer needle, the tip position was once more adjusted under CT scan and the tissue sample was again obtained. The operator repeated this procedure until sufficient amount of tissue for histopathological diagnosis was obtained. Specimens were obtained from individual patients; and each specimen was placed in an individual 10% formalin-filled container. The baseline characteristics of the lesions and procedures are summarized in Table 1. Immediately after biopsy, unenhanced CT scan (slice thickness, 10 mm) of the whole thorax was performed to check for immediate pneumothorax. If immediate development of small, asymptomatic pneumothorax was observed, the patient was treated conservatively by administration of supplemental oxygen. Follow-up chest radiography was performed to evaluate the stability of pneumothorax. Any immediate occurrence of a moderate pneumothorax, defined as a collapse of the lung surface ≥ 2 cm from the site of the needle puncture, was aspirated by reinsertion of the coaxial needle guide. Patients were given chest tube insertion if the pneumothorax worsened or was accompanied by symptoms such as respiratory distress, shortness of breath, pain and decreased oxygen saturation. Patients without any complications or with stable, mild complications were discharged after 24 hours of observation. The discharged patients were instructed to return to the nearest emergency department upon development of symptoms such as substantial pain and shortness of breath.
One radiologist reviewed the pathology reports of biopsy specimens, follow-up images and surgical specimens of patients, who underwent surgical resection. Lesion changes over time were investigated on follow-up images and the mean follow-up period was 13 months (range: 7-20 months) in our study. Final diagnosis was confirmed in four ways. First, if the patient had underwent surgical resection (n = 11) for the pleural lesion, final diagnosis was considered to be matching with the pathologic reports of the surgical specimen. Second, if a malignant or specific benign pathologic abnormality (e.g., mesothelioma and TB) (n = 64) was reported because of biopsy, then the biopsy result was accepted as the final diagnosis. Third, if a nonspecific benign pathologic abnormality (e.g., negative for malignancy or chronic inflammation) was reported as a result of biopsy and there was at least a 20% decrease in lesion diameter or the lesion was stable (n = 9) during the follow-up of more than 1 year on chest radiograph or CT scan, then the final diagnosis was recorded as benign. Finally, if a nonspecific benign pathologic abnormality was reported as a result of biopsy, but the lesion did not exhibit sufficient interval decrease in size or could not be tracked by follow-up images, then its final diagnosis was defined as undetermined (n = 4).
Using the final histological diagnoses and the clinical and radiological course of the diseases as reference, positive biopsy results were categorized as true-positive or false-positive, if the final diagnosis was malignant or benign disease, respectively. Similarly, negative biopsy results were categorized as true-negative or false-negative, in case the final diagnosis was benign or malignant disease, respectively. Subsequently, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the CNB diagnoses and the overall diagnostic accuracy were calculated. Undetermined nodules were not included in the calculation of diagnostic accuracy, sensitivity and specificity (9).
A statistical software package (version 16.0, SPSS Inc., Chicago, IL, USA) was used for analysis followed by a review and full collaboration and clinical feedback from radiologists. To evaluate the effect of influencing factors for diagnostic accuracy and complications in CT-guided CNB for pleural lesions, univariate analysis using the independent sample Student's t test for continuous values and the Fisher's exact test for categorical values were performed. Subsequently, for variables, where univariate analysis showed a p value less than 0.05, the samples were subjected to multivariate logistic regression analysis (enter method) in order to determine the independent influencing factors for diagnostic accuracy and complications. A p value of less than 0.05 was determined to indicate statistical significance.
The pathological diagnoses, which were determined by a CT-guided CNB of pleural lesions and various methods for final diagnoses, are detailed in Table 2. CNB diagnoses included 28 benign, 56 malignant and four undetermined cases. Final diagnoses consisted of nineteen benign, 65 malignant and four undetermined cases. The comparative analysis of histology of image guided pleural biopsies with outcomes is provided in Table 3. The accuracy, sensitivity, specificity, PPV, and NPV for CT-guided CNB of pleural lesions were 89.2%, 86.1%, 100%, 100%, and 67.8%, respectively. The final diagnoses of the false-negative biopsies were sarcomatoid carcinoma in four cases, adenocarcinoma in three cases and atypical carcinoid tumour in two cases. Two patients had metastatic sarcomatoid carcinoma confirmed via cervical lymph node biopsy. One patient underwent mediastinal mass excision, which confirmed a mediastinal atypical carcinoid tumor. Whereas, other patients underwent surgical resection, which confirmed a diagnosis of sarcomatoid carcinoma and adenocarcinoma. There were no false-positive malignant results. Results from the univariate analyses are summarized in Table 4, which provides insight into diagnostic accuracy. The probable influencing factors such as age (p = 0.510), sex (p = 0.118), lesion size (p = 0.078), pleural-puncture angle (p = 0.695), pleural effusion (p = 1.000) did not have a significant influence on accuracy.
In terms of procedure-related complications, pneumothorax was detected in 14 patients (16.0%) and chest pain in 2 patients (2%). No incidence of hemoptysis or deaths was noted in the study group. However, hemothorax was detected in one (1%) patient after biopsy; which later was hemodynamically stable and did not required transfusion. There were significant differences between the pneumothorax group and the non-pneumothorax group in terms of lesion size/pleural thickness (cm) (p = 0.029), pleural effusion (p = 0.027) and the number of pleural punctures (p = 0.011) (Table 5). Multivariate analysis revealed lesion/pleural thickening size as a significant independent risk factor (odds ratio [OR], 8.744; 95% confidence interval [CI], 1.957-39.073; p = 0.005), and the presence of pleural effusion as a significant independent protective factor (OR, 0.171; 95% CI, 0.033-0.901; p = 0.037) (Table 6). Factors such as patient's age, sex, pleural-puncture angle, patient position, and number of pleural punctures were not associated with an increased risk of pneumothorax.
Computed tomography scan provides excellent contrast and spatial resolution and enables accurate needle placement making it the most widely used guidance technique for percutaneous transthoracic interventional procedures. The results from the present study propose CT as a safe and effective guidance modality for the biopsy of pleural lesions. However, the use of ionizing radiation, lack of real-time capability and visualization of only transverse sections are major limitations of CT-guided pleural biopsy.
The overall sensitivity of 86.1% for the 88 pleural lesions in our study is essentially equal to the sensitivities reported in previous studies (10, 11, 12) and the rate of non-diagnostic pleural biopsies (5%) also comparable with prior research (13). In cases of CNB diagnoses, when a malignant diagnosis is identified by needle biopsy, the clinical decision-making process is generally straightforward due to the extremely low false-positive rates (14). However, when a benign diagnosis is obtained, there is a clinical uncertainty over how to proceed, because a number of these lesions may prove to be malignant (false negatives). Accordingly, it is recommended that patients with benign CNB diagnoses undergo repeat imaging within one or more years in order to document the stability or resolution of the pleural lesions. If the lesion grows in size, a repeat biopsy or VATS may be required to obtain a definitive diagnosis (15).
There are several factors, which possibly contributes to the diagnostic advantage of CT-guided pleural biopsy, as seen in this study. Primarily, the ability of imaging to ensure that the biopsy specimen is taken from an area of abnormal pleural tissue is certainly the obvious one. Results of observational series have suggested a substantial improvement in quality of samples in situations where CT-guided biopsy is employed. Second, CT-guided CNB technique also revealed its effectiveness in cases where pleural effusion was present and we have also used it in cases of thin pleural thickening. To achieve adequate diagnostic samples in patients with thin (0.5-1 cm) pleural thickening, we used a tangential approach, which proved its effectiveness in gathering biopsy tissues. Third, the accuracy for diagnosis of pleural lesion with CT-guided CNB in this study (89.2%) is only slightly lower than the published sensitivities from thoracoscopy series (91-95%) (6, 7). Accordingly, it is hypothesized that this technique might be used as an alternative in the absence of thoracoscopy or if the patient is unfit for thoracoscopy.
Generally, it is implicited that pneumothorax is the most common complication of lung needle biopsy (16, 17). In our study, the pneumothorax rate (16%) and consequential chest tube insertion rate (2%) of CT-guided CNB for pleural lesion were within the range of those in prior publications (10, 12, 18). Multivariate analysis showed an increased risk of pneumothorax with decrease in lesion size, as also reported by previous studies, and a correlation between reduced lesion sizes with a higher number of complications was noted. In the case of pneumothorax, this may be due to the number of pleural needle passes required to obtain correct needle direction, which as a result, increases the number of minor pleural injuries. On the other hand, the presence of pleural effusion significantly reduced the occurrence of pneumothorax. It is assumed that pleural effusion may seal off the needle tract and block air leakage into the pleural space, which, in turn can contribute to a decrease in the pneumothorax rate.
According to a study by Sconfienza et al. (18), US guidance is comparable to CT guidance in terms of sample accuracy and allows for a significant reduction in procedure time and post-procedural pneumothorax. In addition, it does not require ionizing radiation. However, there is still no randomized study on comparison between diagnostic yields of ultrasound-guided with CT-guided pleural biopsy.However, reported figures suggest that the latter may have a marginally higher yield (12, 19, 20, 21, 22, 23, 24, 25). Currently, all imaging-guided thoracic biopsies at our institution are routinely performed under CT guidance. For abdominal biopsy, we favor the use of US guidance because it is faster, easier and less cumbersome. Therefore, the outcome of this study is manifested to be valuable for clinical practice.
There are certain limitations to this study. First, it is a retrospective analysis and preconceived notion may have existed. Second, the individual radiologist's preference for a needle path and angle (based on their level of expertise)was difficult to evaluate. Third, four of 88 lesions (5%) were regarded as undetermined. Fourth, our study did not include very small pleural lesions/pleural thickening (< 5 mm).
In conclusion, it is appropriate to state CT-guided core needle biopsy of pleural lesion as a safe procedure with high diagnostic yield and low risk of significant complications.
Figures and Tables
Fig. 1
54-year-old man with multiple pleural-based nodules and right sided pleural effusion undergoing CT-guided biopsy for left anterior nodular pleural lesion measuring 0.8 mm. Histopathology of specimen revealed pleural fibrosis.
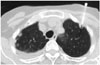
Fig. 2
46-year-old man with bilateral diffuse pleural thickening undergoing CT-guided biopsy through posterior approach. Pleural thickening at biopsy site was measured to be 10 mm. Histopathology of specimen revealed spindle cell carcinoma.
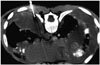
Table 1
Characteristic of 88 Pleural Lesions and Technical Factors of CT-Guided Core Needle Biopsy Procedures

Table 2
Results of CT-Guided CNB and Final Diagnosis for Pleural Lesions
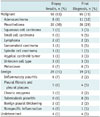
Table 3
Positive and Negative Diagnosis

| CNB Diagnosis | Final Diagnosis | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 56 | 0 | 56 |
| Negative | 9 | 19 | 28 |
| Total | 65 | 19 | 84 |
Table 4
Results of Univariate Analysis Used to Determine Risk Factors Associated with Diagnostic Failure
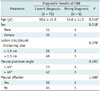
Table 5
Results of Univariate Analysis to Determine Influencing Factors for Pneumothorax
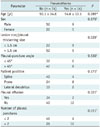
Table 6
Pneumothorax Rate According to Various Related Factors Evaluated by Multivariate Logistic Regression

References
1. Feragalli B, Storto ML, Bonomo L. Malignant pleural disease. Radiol Med. 2003; 105:266–288. quiz 289-290.
2. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007; 188:37–41.
3. Mohammadi A, Oshnoei S, Ghasemi-rad M. Comparison of a new, modified lung ultrasonography technique with high-resolution CT in the diagnosis of the alveolo-interstitial syndrome of systemic scleroderma. Med Ultrason. 2014; 16:27–31.
4. He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014; 192:553–561.
5. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009; 50:42–47.
6. Froudarakis ME. New challenges in medical thoracoscopy. Respiration. 2011; 82:197–200.
7. Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J. 2003; 22:589–591.
8. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011; 16:738–746.
9. Kim GR, Hur J, Lee SM, Lee HJ, Hong YJ, Nam JE, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol. 2011; 21:232–239.
10. Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001; 219:510–514.
11. Adams RF, Gray W, Davies RJ, Gleeson FV. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest. 2001; 120:1798–1802.
12. Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003; 361:1326–1330.
13. Scott EM, Marshall TJ, Flower CD, Stewart S. Diffuse pleural thickening: percutaneous CT-guided cutting needle biopsy. Radiology. 1995; 194:867–870.
14. Gelbman BD, Cham MD, Kim W, Libby DM, Smith JP, Port JL, et al. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol. 2012; 7:815–820.
15. Lee IJ, Bae YA, Kim DG, Jung KS, Im HJ, Lee K, et al. Percutaneous needle aspiration biopsy (PCNAB) of lung lesions: 5 years results with focusing on repeat PCNAB. Eur J Radiol. 2010; 73:551–554.
16. Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011; 196:W678–W682.
17. Wu RH, Tzeng WS, Lee WJ, Chang SC, Chen CH, Fung JL, et al. CT-guided transthoracic cutting needle biopsy of intrathoracic lesions: comparison between coaxial and single needle technique. Eur J Radiol. 2012; 81:e712–e716.
18. Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 2013; 266:930–935.
19. Stigt JA, Boers JE, Groen HJ. Analysis of "dry" mesothelioma with ultrasound guided biopsies. Lung Cancer. 2012; 78:229–233.
20. Chang DB, Yang PC, Luh KT, Kuo SH, Yu CJ. Ultrasound-guided pleural biopsy with Tru-Cut needle. Chest. 1991; 100:1328–1333.
21. Heilo A, Stenwig AE, Solheim OP. Malignant pleural mesothelioma: US-guided histologic core-needle biopsy. Radiology. 1999; 211:657–659.
22. Diacon AH, Schuurmans MM, Theron J, Schubert PT, Wright CA, Bolliger CT. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration. 2004; 71:519–522.
23. Koegelenberg CF, Bolliger CT, Theron J, Walzl G, Wright CA, Louw M, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax. 2010; 65:857–862.
24. von Groote-Bidlingmaier F, Koegelenberg CF, Bolliger CT, Chung PK, Rautenbach C, Wasserman E, et al. The yield of different pleural fluid volumes for Mycobacterium tuberculosis culture. Thorax. 2013; 68:290–291.
25. Metintas M, Ak G, Dundar E, Yildirim H, Ozkan R, Kurt E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest. 2010; 137:1362–1368.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download