Abstract
MR imaging appearances of different types of reconstructive muscle flaps following reconstructive surgery of the lower extremity with associated post-surgical changes due to altered anatomy, radiation, and potential complications, can be challenging. A multidisciplinary therapeutic approach to tumors allows for limb salvage therapy in a majority of the patients. Decision-making for specific types of soft tissue reconstruction is based on the body region affected, as well as the size and complexity of the defect. Hematomas and infections are early complications that can jeopardize flap viability. The local recurrence of a tumor within six months after a complete resection with confirmed tumor-free margins and adjuvant radiation therapy is rare. Identification of a new lesion similar to the initial tumor favors a finding of tumor recurrence.
A multidisciplinary approach comprising of orthopedic and reconstructive surgery, oncology, radiotherapy, and radiology, coupled with advances in the surgical management of complex soft tissue defects has led to an increase in limb salvage resections of soft tissue and bone tumors, as a viable alternative to amputation, without compromising outcomes and without increased risk of recurrence (1, 2, 3, 4, 5, 6). While this approach allows limb sparing surgery in over 90% of the cases (7), it does make the accurate interpretation of follow-up studies more challenging and crucial. Popov et al. (8) showed that the mean time between resection and local recurrence was 16 months (range 7-90 months) and the recurrence-free survival rate was 84%. Follow-up imaging therefore, requires careful attention, not only to look for tumor recurrence, but also to be aware of the imaging appearances and potential complications of soft tissue reconstructions. We aimed to improve the understanding of the neoanatomy of reconstructed muscle flaps of the lower extremity, their expected post-surgical and post-radiation changes, and common complications in order to improve the accuracy of interpretation.
Close to 75% of musculoskeletal sarcomas occur in lower extremity (4). Preoperative assessments of patients with bone and soft tissue tumors include imaging in order to define the extent of the tumor, presence or absence of metastases, and assess the arterial supply of potential donor sites for subsequent soft tissue flap reconstruction. Tumor excision is planned by the orthopedic oncology surgical team. Reconstructive surgery is planned by the reconstructive surgery team with respect to the anticipated defect resulting from the tumor excision and the availability and proximity of a donor flap. After complete macroscopic resection of the tumor and preliminary wound closure, confirmation of the tumor-free resection margins by pathology conducted. Immediate reconstruction should be pursued whenever possible: delayed reconstruction should be limited to cases where there is uncertainty about the tumor margins. In these cases in general, radiation therapy should start one month after reconstructive surgery and lasts for 4 to 8 weeks. Follow-up tumor surveillance imaging is usually performed at 6, 12, and 18 months following surgery.
Wound closure can be achieved either by direct closure, skin graft, or by free or local flaps. There are a wide variety of flaps available for consideration, and the choice is often patient-specific, based on the complexity and size of the defect, its location, and the availability of local vascularized donor tissue and anticipated cosmetic outcome (3, 4, 5, 9). The primary aim of the flap is to provide adequate coverage of the exposed neurovascular structures, bone, allograft, and tumor prosthesis. Local flaps provide coverage of the local vascularized tissue adjacent to the defect. The donor site for free flaps is remote to the surgical defect, necessitating multiple surgical sites and often, longer operating times. Whenever possible, local flaps are preferred over free flaps (10). Each region has unique characteristics resulting in various reconstruction techniques (11, 12, 13).
In most cases, the pelvis and thigh offer enough soft tissue volume and several potential long donor muscles which are amenable to adequate mobilization and rotation to cover the defects without compromising vascularity. Common donor muscles include the tensor fascia lata (Fig. 1), biceps femoris, rectus femoris, and the gluteus muscles (Fig. 2) (11, 12, 13).
Similar to the pelvis and proximal thigh, local pedicle flaps are commonly used in the mid-thigh. To achieve wound closure, the tensor fascia lata, vastus lateralis, rectus femoris, and gracilis muscle are feasible options (Fig. 3) (11, 12, 13). When the blood supply to potential local flaps is compromised during tumor resection, a free flap like the latismus dorsi muscle could also be used (9).
Defects in this region are usually covered by local pedicle flaps using either the lateral or medial heads of the gastrocnemius muscle. With larger defects, closure can be obtained by combining the lateral or medial heads of the gastrocnemius muscle with the whole or partial soleus muscle (Fig. 4) (10, 11, 12, 13).
Soft tissue reconstruction, especially involving the weight-bearing plantar surface, is required to tolerate significant levels of mechanical stress, which may be difficult to achieve. Thus, in these cases, pedicle flaps with better vascularization and lower risk of failure (like the distal sural pedicle flap used for covering the plantar defect over the heel) are preferred. In situations with lower mechanical stress, a free flap is also amenable (Fig. 5) (10, 11, 12, 13).
Complications following soft tissue reconstructive surgery are uncommon (12, 14, 15). Hematomas or fluid collections (Fig. 8) and infections (Fig. 9) are more likely to be encountered when compared to other complications, such as graft necrosis, graft failure, and local recurrence (5).
The MRI features of hematomas can be very variable and depend on the age of the bleed. Some can mimic fluid collection (low signal intensity in T1-weighted and high signal intensity in T2-weighted sequences) and others, like subacute and long standing hematomas, can demonstrate increased signal on T1-weighted images. Contrast-enhanced MRI is often needed to differentiate a complex hematoma from a recurrent tumor. Hematomas demonstrate characteristic peripheral enhancement with a non-enhancing core, while tumors demonstrate nodular enhancement (16). Seroma is a common complication following soft tissue sarcoma surgery. Postoperative seromas are soft tissue lesions with well-defined margins in the surgical bed, demonstrating a low or intermediate T1-weighted signal intensity relative to adjacent muscle and a very high signal intensity on T2-weighted images (17). Some seromas contain focal nodular areas of low-to-intermediate signal within them. These are more likely to represent organized hematomas. Local recurrence is very rare within seromas (18).
The muscle portion of the myogenous flaps, such as rectus abdominis and latissimus dorsi, can undergo atrophy post operatively, with the MRI revealing a loss of muscle volume (19). Infections can damage the flap by direct extension and by increasing metabolism, leading to flap necrosis (12, 15).
Care should be taken to differentiate radiation-induced changes from infection, which also demonstrates edema and contrast enhancement involving the flaps but no focal mass effect (Fig. 10). The latter tend to be more diffuse, while radiation-induced changes follow the size and shape of the radiation portal. Additionally, correlation with clinical data is essential. Sometimes, a needle-guided aspiration may be necessary to differentiate the two. In tumor patients, close scrutiny should be provided regarding tumor recurrence, depicted as new, mass-like, nodular, expanding lesions that demonstrate contrast enhancement, especially in the first 6-12 months after resection (Fig. 11) (7, 8). The comparison of the imaging pattern of the initial tumor and the recurrence can be very helpful to differentiate these from complex fluid collection. In ambiguous cases, dynamic imaging with a rapid injection of contrast may be helpful because recurrent tumors tend to enhance more rapidly than inflammation, or fibrosis (20). If the finding still remains suspicious, a needle-guided aspiration may be necessary to differentiate.
Our imaging protocol on a 1.5-T Magnet (Signa, GE, Milwaukee, WI, USA), includes several phases.
First, a coronal short tau inversion recovery sequence, which is sensitive for fluid, depicts edema and fluid collection, and allows a quick overview of the existing pathology. The disadvantages are the low resolution and inability to depict anatomical details. The coronal imaging plane reduces the number of images to a minimum and covers large areas in a minimum amount of time. It can be used to plan the following sequences and restrict the focus to the necessary areas, thus reducing imaging time.
Next, an axial and sagittal T2-weighted fast spin echo with fat saturation are performed. These two sequences are sensitive for fluid too, but allow a better depiction of anatomical details compared to the first sequence. A major limitation is the susceptibility to artifacts caused by metal implants, surgical clips, and sutures. Another disadvantage is an inhomogeneous fat suppression, especially in the forearm, hand, and the foot.
We also perform an axial proton density and T1-weighted sequence. These sequences provide excellent spatial resolution for a detailed assessment of anatomical structures, such as skin, subcutaneous tissue, muscles, ligaments and tendons, vessels, and nerves.
Finally, we conduct an axial spoiled gradient recalled sequence with fat saturation without and with intravenous gadolinium contrast. In comparing the pre- and post-contrast images, small areas of enhancement can be assessed allowing, for example, the detection of local recurrence, infection, and tissue necrosis. All sequences should cover both adjacent joints next to the primary region of interest. The covered volume is adjusted individually.
In patients with suspected osteolytic changes, radiography and computed tomography (CT) are viable methods. While radiography allows for a fast overview of bony structures, CT allows for a better assessment of endosteal and cortical osteolytic bony changes. The disadvantage of CT is the susceptibility to metal artifacts with loss of interpretability of structures around larger metallic implants. MR imaging is also sensitive to cortical changes, but it is the method of choice to depict bone marrow changes indicating possible tumor recurrence (21). Again, a major limitation is the susceptibility to artifacts caused by metal implants, surgical clips, and sutures.
Recent advantages in reconstructive surgery allow for different types of wound closure depending on location, size, mechanical requirements, and availability of donor tissue either locally or from other body parts. Knowledge of imaging appearances of different types of reconstructive muscle flaps with associated postsurgical changes of anatomy and differentiation of potential complications is essential and optimizes patient management.
Figures and Tables
Fig. 1
63-year-old woman with right groin high-grade fibromyxoid sarcoma with extensive central necrosis and tensor fascia lata myocutaneous flap following tumor resection.
A. Preoperative axial gadolinium-enhanced T1-weighted MR image with fat saturation depicts tumor (T). Largely non-enhancing myxoid tumor infiltrates skin and underlying sartorius muscle (*) with loss of fat plane at tumor-muscle interface. B. Axial proton density-weighted MR image 6 months after surgery and radiation. Note postsurgical anteromedial shift of tensor fascia lata muscle (M) and coverage of soft tissue defect by tensor fascia lata myocutaneous flap (arrows). Fem = femur
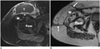
Fig. 2
57-year-old paraplegic female with right ischial osteomyelitis and pressure ulcer, underwent partial ischiectomy and wound closure with gluteus maximus muscle flap.
A. Preoperative axial gadolinium enhanced T1-weighted image with fat saturation. Note periostal reaction and loss of cortical outline of right ischium (*). Also, note extensive right gluteal hyper intensity interpreted as edema and abscess (arrow) posterolateral to right ischium (*). B. Postoperative coronal T1-weighted image. Persistent changes of osteomyelitis (*) show low signal in right ischium. C. Postoperative axial T2-weighted image with fat saturation. Note inferomedial relocation of gluteus maximus muscle (M) on right, resulting in significant soft tissue asymmetry (involving both muscle and fat) compared to normal left ischial region. D. Postoperative axial T1-weighted image at same level showing resolving ischial bone changes (*) following additional antibiotic therapy. S = symphysis pubis
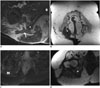
Fig. 3
39-year-old man with proximal inner thigh fibromyxoid sarcoma and wound closure with myocutaneous gracilis flap following tumor resection.
A. Axial proton density-weighted MR image after incomplete resection showing scar (arrow) on inner thigh. Markers (*) were placed on skin surface adjacent to scar. B. Axial T1-weighted MR image following complete re-resection shows relocation and stretching of gracilis muscle (M) and subcutaneous fat (arrows) which have been shifted to cover surgical defect. Fem = right femur
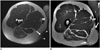
Fig. 4
30-year-old woman with excision of high-grade myxofibrosarcoma and wound closure with lateral gastrocnemius flap.
A. Axial proton density-weighted MR image shows tumor (T) with infiltration of skin and iliotibial band of knee (*). Cutaneous marker (arrow). B. Axial gadolinium enhanced T1-weighted MR image with fat saturation shows vascular pedicle (arrow) of gastrocnemius flap (F). C. Axial proton density-weighted MR image more proximally at level of lateral femoral condyle (C) shows the flap (F) covering lateral post-excisional defect. D. Coronal short tau inversion recovery image shows craniocaudal extension of flap (F). Note edema of skin (arrows) following radiation therapy.

Fig. 5
25-year-old man with crush injury of lower leg, comminuted calcaneus fracture, and compartment syndrome. After fasciotomy, delayed closure of defect with free gracilis flap was performed.
A. Coronal T1-weighted MR image shows extent (arrows) of gracilis muscle flap (F) in medial and plantar aspects of midfoot. B. Sagittal T2-weighted MR image with fat saturation shows longitudinal extent of flap (F) overlying the proximal plantar foot. C = 2nd cuneiform bone
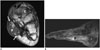
Fig. 6
57-year-old woman with severe crush injury, comminuted fractures of tibia, fibula, and large open wound with exposed tibia extending from upper tibia to ankle. Wound closure was performed with rectus abdominis free flap.
A. Anteroposterior radiograph of lower leg shows multiple fractures of tibia and fibula. Note extensive soft tissue abnormality with anteromedial defect (arrows) and emphysema (*). B. Axial CT scan proximal to fibular fracture. Note soft tissue emphysema (*) anterolaterally and overlying skin defect (arrow). C. Anteroposterior radiograph after soft tissue reconstruction shows prominent lobulated soft tissue medial to distal tibia, which represents soft tissue flap (F). Also note internal fixation of tibial fracture (*) with improved alignment. G = gauze, T = left tibia
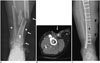
Fig. 7
57-year-old woman with excision of distal lower leg high-grade myxofibrosarcoma and wound closure with latissimus dorsi free flap.
A. Axial T1-weighted MR image after tumor excision. Note large skin defect (arrows) adjacent to fibula (*) with peroneus muscle exposed. B. Axial T1-weighted MR image 1 year after reconstruction with optimal coverage of fibula. Note extensive, but expected, fatty infiltration of muscle flap (F). T = left tibia
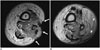
Fig. 8
48-year-old woman after resection of high-grade myxofibrosarcoma and wound closure with lateral gastrocnemius flap.
Coronal T2-weighted MR image (A) and axial T2-weighted MR image (B) with fat saturation. Note fluid collection (*) proximal to flap (F), located at undersurface of graft, extending proximally. arrow = cutaneous marker. C = left lateral femoral condyle, Fem = left femur
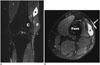
Fig. 9
47-year-old male patient with lower leg high-grade undifferentiated pleomorphic sarcoma and subsequent postsurgical defect covered with combined myocutaneous soleus/medial gastrocnemius flap.
A. Preoperative axial gadolinium enhanced T1-weighted MR image with fat saturation shows enhancing tumor (T) abutting tibia and underlying soleus muscle (M). * = right fibula. B. Post-operative axial gadolinium enhanced T1-weighted MR image with fat saturation shows patchy enhancement of flap (F) and adjacent soft tissue, without mass effect. Even though non-specific, this was clinically interpreted as infection in keeping with clinical presentation and findings.
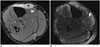
Fig. 10
55-year-old man with myxoid liposarcoma and post-surgical wound covered with lateral gastrocnemius flap after resection. Axial T2-weighted MR image with fat saturation shows homogenous hyperintensity (arrows) of irradiated region with well-defined margins best noted anteriorly. This differs from patchy enhancement (Fig. 9) attributed to infection. There is also some joint effusion in lateral recess of knee joint. Fem = left femur
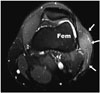
Fig. 11
59-year-old woman with recurrence of high grade myxofibroma after initial wound closure by medial gastrocnemius flap and re-excision closure with free latissimus dorsi flap.
A. Coronal T1-weighted MR image shows lobular and heterogeneous appearance of recurrent tumor (T), which extends proximally and medially to gastrocnemius flap (F). B. Coronal T1-weighted MR image after re-excision and coverage of defect with free latissimus dorsi flap (F), which extends from medial thigh. C. Corresponding axial T1-weighted MR image after re-excision and coverage of popliteal region with free latissimus dorsi flap (F). C = medial femoral condyle, Tib = left tibia
References
1. Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg. 2009; 129:43–49.
2. Drake DB. Reconstruction for limb-sparing procedures in soft-tissue sarcomas of the extremities. Clin Plast Surg. 1995; 22:123–128.
3. Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol. 2006; 94:479–489.
4. Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg. 2009; 62:161–174.
5. Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg. 2009; 62:252–257.
6. Serletti JM, Carras AJ, O'Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg. 1998; 102:1576–1583. discussion 1584-1585.
7. Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg. 1995; 82:1208–1212.
8. Popov P, Tukiainen E, Asko-Seljavaara S, Huuhtanen R, Virolainen M, Virkkunen P, et al. Soft-tissue sarcomas of the upper extremity: surgical treatment and outcome. Plast Reconstr Surg. 2004; 113:222–230. discussion 231-232.
9. Zenn MR, Levin LS. Microvascular reconstruction of the lower extremity. Semin Surg Oncol. 2000; 19:272–281.
10. Parrett BM, Talbot SG, Pribaz JJ, Lee BT. A review of local and regional flaps for distal leg reconstruction. J Reconstr Microsurg. 2009; 25:445–455.
11. Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg. 2001; 108:1029–1041. quiz 1042.
12. Moreira-Gonzalez A, Djohan R, Lohman R. Considerations surrounding reconstruction after resection of musculoskeletal sarcomas. Cleve Clin J Med. 2010; 77:Suppl 1. S18–S22.
13. Achauer B, Eriksson E, Guyuron B, Coleman IJ, Russel R, Vander Kolk C. Plastic surgery: indications, operations, and outcomes. Saint-Louis: Mosby;2000. p. 475–496.
14. Parrett BM, Pribaz JJ, Matros E, Przylecki W, Sampson CE, Orgill DP. Risk analysis for the reverse sural fasciocutaneous flap in distal leg reconstruction. Plast Reconstr Surg. 2009; 123:1499–1504.
15. Peat BG, Bell RS, Davis A, O'Sullivan B, Mahoney J, Manktelow RT, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994; 93:980–987.
16. Trecek J, Sundaram M. Radiologic case study. Extensive hematoma of the vastus intermedius showing components of subacute and chronic hemorrhage with associated myositis of the vastus intermedius and medialis. Orthopedics. 2007; 30:806880–881.
17. Varma DG, Jackson EF, Pollock RE, Benjamin RS. Soft-tissue sarcoma of the extremities. MR appearance of post-treatment changes and local recurrences. Magn Reson Imaging Clin N Am. 1995; 3:695–712.
18. Poon-Chue A, Menendez L, Gerstner MM, Colletti P, Terk M. MRI evaluation of post-operative seromas in extremity soft tissue sarcomas. Skeletal Radiol. 1999; 28:279–282.
19. Ferguson PC. Surgical considerations for management of distal extremity soft tissue sarcomas. Curr Opin Oncol. 2005; 17:366–369.
20. Vanel D, Lacombe MJ, Couanet D, Kalifa C, Spielmann M, Genin J. Musculoskeletal tumors: follow-up with MR imaging after treatment with surgery and radiation therapy. Radiology. 1987; 164:243–245.
21. Costelloe CM, Kumar R, Yasko AW, Murphy WA Jr, Stafford RJ, Lewis VO, et al. Imaging characteristics of locally recurrent tumors of bone. AJR Am J Roentgenol. 2007; 188:855–863.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download