Abstract
Objective
Bilateral oophorectomy leads to reduced bone mineral density (BMD), and reduced BMD is associated with increased marrow fat and reduced marrow perfusion. Purpose of this study was to investigate how soon these changes occur following surgical oophorectomy.
Materials and Methods
Six patients who underwent hysterectomy and bilateral salpingo-oophorectomy were studied. At baseline, mean patient age was 49.5 years (range: 45-54 years). Third lumbar vertebral body BMD measurement using quantitative CT, marrow fat fraction (FF) using MR spectroscopy and marrow perfusion using dynamic contrast enhanced MRI were conducted immediately prior to surgery and at 3, 9, and 21 months after surgery.
Results
Reduced BMD, increased marrow FF, and reduced marrow perfusion occurred synchronously post-oophorectomy. There was a sharp decrease of 12.5 ± 7.2% in BMD (n = 6), a sharp increase of 92.2 ± 46.3% (n = 6) in FF, a sharp decrease of 23.6 ± 3.9% in maximum contrast enhancement (n = 5), and of 45.4 ± 7.7% for enhancement slope (n = 5) during the initial 3 months post surgery. BMD and marrow perfusion continued to decrease, and marrow FF continued to increase at a slower rate during the following 18 months. Friedman test showed a significant trend for these changes (p < 0.05).
Bilateral oophorectomy causes a systemic reduction in bone mineral density (BMD) in both, experimental animals and female human subjects (1, 2, 3, 4). Lumbar spine BMD shows only a small age-related decrease before menopause, but a large change after menopause (1). Measurement of spine BMD by quantitative CT (QCT) in young women with estrogen deficiency averaged 20% below the expected value for age (5, 6, 7). Both men and women experience an increase in marrow fat content and a decrease in bone marrow perfusion with increased age, as well as with decreasing BMD (8, 9, 10, 11, 12, 13). Endothelial dysfunction is a potential cause of impaired bone perfusion in osteoporosis (14, 15). Surgical oopherectomy without ovarian hormone replacement in monkeys showed coronary artery vasoconstriction after an acetylcholine infusion, whereas their counterparts with physiologic replacement of estradiol had vasodilation after the acetylcholine infusion. Co-administration of progestin attenuated vasoconstriction (16).
The temporal relationship between reduced BMD, reduced marrow perfusion, and increased bone marrow fat, and how quickly these changes occur after female sex hormone depletion remains unknown. MR proton (1H) spectroscopy (MRS) is used to quantify marrow fat content, while dynamic contrast-enhanced (DCE) MR imaging is used to assess bone marrow perfusion (8, 9, 10, 11, 12, 13, 17, 18, 19). The reproducibility of these techniques is sufficiently high to allow for serial evaluation of either longitudinal changes of marrow fat content and marrow perfusion (19). We undertook this longitudinal study to investigate the temporal relationship between BMD, marrow fat content and bone marrow perfusion after bilateral oophorectomy in women using MR imaging.
The study was approved by the Institutional Ethics Committee and all subjects provided informed signed consent. In total 6 female patients with a mean age of 49.5 years (range: 45-54 years) were studied. Five patients had menorrhagia due to uterine fibroids and uterine adenomyosis occurred in the remaining case. These patients had no malignancies or hematogical disorders, and had no medication that could lead to osteoporosis. The body mass index of the patients ranged from 17.9 to 28.3 (mean: 24.7) kg/m2. Hysterectomy plus bilateral salpingo-oophorectomy was performed in all cases with uneventful surgical recovery. BMD measurement and MRS measurement was conducted in all 6 patients at baseline and 3 months post surgery; while 5 patients completed DCE MRI measurement at baseline and 3 months after surgery. At 9 months after surgery, 5 patients completed BMD measurement and MRS measurement, while 4 patients completed DCE MRI measurement. At 21 months post surgery, 4 patients had MRS measurement, 3 patients had BMD measurement and 2 patients had DCE MRI measurement.
The third lumbar (L3) vertebra trabecular BMD was measured using a multidetector CT (LightSpeed VCT 64, General Electric, Milwaukee, WI, USA). A QCT Torso Phantom was used as the external reference. QCT 5000 bone densitometry software (Image Analysis Inc., Columbia, KY, USA) was used to calculate BMD values. MRI was performed on a 3-tesla clinical system (Achieva, Philips Healthcare, Best, the Netherlands). A surface coil was placed under the lumbar spine region as the radiofrequency receiver and the body volume coil was used as the radiofrequency transmitter. Sagittal images of the lumbar spine were obtained to guide positioning of L3 vertebral body. The width (w), depth (d), and height (h) of the L3 vertebral body were measured on MR images to define a volume of interest. A volume of interest with dimensions w/2·d/2·h/2 cm3 was located central to the vertebral body. After local shimming and gradient adjustments, data were acquired at a spectral bandwidth of 1000 Hz and with 512 data points, and 64 non-water suppressed signals were obtained by using a point-resolved MR spectroscopic sequence (repetition time [TR]/echo time [TE] = 3000/25) (9, 10).
MR spectroscopic data were analyzed at an off-line computer (Precision 650 Workstation; Dell, Austin, TX, USA). Water (4.65 ppm) and lipid (1.3 ppm) peak amplitudes were measured to determine vertebral marrow fat fraction (FF), which was defined as the relative fat signal amplitude in terms of a percentage of total signal amplitude (water and fat). It was calculated according to the following equation: fat content = (Ifat / [Ifat + Iwat]) × 100, where Ifat and Iwat are the peak amplitudes of fat and water, respectively. No correction for relaxation losses was applied (9, 10).
For DCE MRI, after obtaining an axial T1-weighted image of L3 vertebra, a dynamic short T1-weighted gradient echo sequence single slice MR series was obtained in the axial plane using the following parameters: TR = 4.2 msec, TE = 2.3 msec, flip angle = 12°, slice thickness = 10 mm, matrix = 300 × 74, in-plane resolution = 1.0 × 2.0 mm2, number of excitation = 1. A bolus of gadoteric acid (Dotarem; Guerbet, Roissy, France) of 0.6 mmol/kg was injected at a rate of 2.5 mL/sec by an MR injection system (Spectris, Medrad, Pittsburgh, PA, USA) through a 21-G intravenous catheter inserted into an antecubital vein, followed by a 20-mL saline flush. Perfusion MRI started after the first 50 image acquisitions.
Dynamic contrast-enhanced MR images were processed on a dedicated workstation (Viewforum, Philips Healthcare). A region-of-interest was drawn over the cancellous part of the L3 vertebra just inside the cortical margins. Signal enhancement over time was recorded, and plotted as a time-signal intensity curve. From the time-signal intensity curve, 2 MR perfusion indices were analyzed, namely, maximum enhancement (Emax) and enhancement slope (Eslope). Emax was defined as the maximum percentage increase of signal intensity from baseline. Eslope was defined as the rate of enhancement between 10% and 90% of the maximum signal intensity difference between maximum signal intensity (Imax) and baseline signal intensity (Ibase), i.e.,
Where Ibase was defined as the mean signal intensity of the first 50 images, Imax was defined as the maximum value of the first rapidly rising part of the time-signal intensity curve, t10% and t90% were the time points at which signal intensity reaches 10% and 90% of the signal intensity difference between Ibase and Imax, respectively (9, 10, 11).
The quantitative results were expressed as mean ± standard deviation. Non-parametric Friedman test was used to test the difference among different examination time points. Statistical analysis was performed using SPSS v.18.0 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered as statistically significant.
No vertebral fracture or other spinal disorder was present in any patient. The longitudinal results of BMD, vertebral marrow FF, Emax, and Eslope were shown in Table 1 and Figures 1, 2, 3, 4, 5, 6. There was a sharp decrease in BMD, a sharp increase in marrow FF, and a sharp decrease in Emax, and Eslope during the initial 3 months followed bilateral oophorectomy. BMD and marrow perfusion continued to decrease, and marrow FF continued to increase, though at a slower rate during the later follow-up period. These changes were concomitant. Friedman test showed a statistically significant trend among different examination time points (Table 2).
Bone resorption increases more suddenly after an artificial rather than natural menopause, because of the acute decrease in serum estradiol. While the rate of bone loss in the peri- and post-menopausal period can be up to 5% per annum, a prospective study showed that lumbar vertebral trabecular bone decreased by 15% to 19% during the first 12 months after bilateral oophorectomy; however, within several years the rate of trabecular bone loss slowed to the more typical post-menopausal rate of 1% per year (20). Prior et al. (21) also showed rapid bone loss of 14% in the lumbar spine over one year in oophorectomised women.
Several studies indicated that marrow perfusion is reduced in osteopenic and osteoporotic bone (8, 9, 10, 11, 12). The current study in human subjects concurs with a previous animal-based study that showed a reduction in bone perfusion synchronous with a reduction in BMD post-oophorectomy (22). The reduction in perfusion associated with decreased BMD post-oophorectomy is most likely due to a combination of reduced erythropoetic marrow and endothelial dysfunction (22). An increase in marrow fat is essentially a marker of reduced erythropoetic marrow (13). Positron emission tomography imaging study has indicated that the metabolic activity of erythropoetic marrow is up to 6 times greater than that of fatty marrow (23). The post-oophorectomy reduced bone perfusion may well reflect reduced marrow demand secondary to a reduced red cell mass. Bone metabolism is relatively low compared to functioning marrow, hence a change in bone metabolism rate is unlikely to contribute to changes in marrow perfusion (8, 20, 21, 22, 23). Vertebral bone marrow perfusion significantly decreased in subjects older than 50 years in a cross-sectional study (24). Particularly, women demonstrated a more marked decrease than men older than 50 years (24). The increase of FF in bone marrow, and the associated reduction of red marrow may also be associated with 'senile anemia' where elderly subjects tend to be prone to borderline anemia (25, 26). Two other potential etiologies need to be considered for post-oopherectomy marrow changes. The first is a potential estrogen-mediated switch in mesenchymal stem cell differentiation. A lack of estrogen leads to adipocytic rather than hematopoietic or osteoblastic differentiation of mesenchymal stem cells (27). A reduced need for hematopoietic marrow with cessation of menorrphagia following hysterectomy may have led to conversion to a more fatty marrow in our patient cohort.
The main limitation of the current study was the small patient number, and missed examinations in some patients. Many patients were unwilling to commit to attending multiple times. Technical improvements in quantitative imaging, such as non-invasive arterial spin labeling allow more patient-friendly studies (28, 29, 30). Despite the small number of patients, this was the first longitudinal study of post-oophorectomy patients with serial BMD, marrow fat and marrow perfusion measurements. Despite the small patient number, Friedman test confirmed a statistically significant trend for the observed changes. An age-related conversion of red to yellow bone marrow in the axial skeleton in female subjects begins at 40-49 years of age (31). Therefore, apart from the initial rapid change of FF during the 3 months after oophorectomy, the latter FF increase may be compounded by the aging effect (31). The biological causes of these changes and their relevance to clinical management warrant further studies.
In conclusion, this study demonstrated a sharp increase of marrow FF and a rapid decrease in bone marrow perfusion during the initial 3 months post bilateral oophorectomy. During the later follow-up period up to 21 months, BMD and bone marrow perfusion continued to decrease, and bone marrow fat content continued to increase, although at a slower rate.
Figures and Tables
Fig. 2
Typical MR spectra presentation of marrow fat fraction increase post oophorectomy. Water peak is at 4.65 ppm and lipid peak is at 1.3 ppm.

Fig. 4
Typical time-intensity enhancement curve at baseline (top trace) and 3 months post oophorectomy (bottom trace). Time-intensity curve post surgery shows reduced Emax and less steep Eslope.

Table 1
BMD, Marrow Fat Fraction, Emax and Eslope Changes after Bilateral Oophorectomy

Table 2
P Values for Comparison of Results among Different Examination Time Points
Acknowledgments
The authors are grateful to the statistical advice by one of the reviewers of this paper.
References
1. Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007; 14(3 Pt 2):567–571.
2. Deng M, Wang YX, Griffith JF, Lu G, Ahuja AT, Poon WS. Characteristics of rat lumbar vertebral body bone mineral density and differential segmental responses to sex hormone deficiency: a clinical multidetector computed tomography study. Biomed Environ Sci. 2012; 25:607–613.
3. Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003; 68:10–18.
4. Deng M, Griffith JF, Zhu XM, Poon WS, Ahuja AT, Wang YX. Effect of ovariectomy on contrast agent diffusion into lumbar intervertebral disc: a dynamic contrast-enhanced MRI study in female rats. Magn Reson Imaging. 2012; 30:683–688.
5. Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990; 301:790–793.
6. Cann CE, Martin MC, Genant HK, Jaffe RB. Decreased spinal mineral content in amenorrheic women. JAMA. 1984; 251:626–629.
7. Wolman RL, Clark P, McNally E, Harries M, Reeve J. Menstrual state and exercise as determinants of spinal trabecular bone density in female athletes. BMJ. 1990; 301:516–518.
8. Shih TT, Liu HC, Chang CJ, Wei SY, Shen LC, Yang PC. Correlation of MR lumbar spine bone marrow perfusion with bone mineral density in female subjects. Radiology. 2004; 233:121–128.
9. Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005; 236:945–951.
10. Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006; 241:831–838.
11. Wang YX, Griffith JF, Kwok AW, Leung JC, Yeung DK, Ahuja AT, et al. Reduced bone perfusion in proximal femur of subjects with decreased bone mineral density preferentially affects the femoral neck. Bone. 2009; 45:711–715.
12. Wang YX, Zhang YF, Griffith JF, Zhou H, Yeung DK, Kwok TC, et al. Vertebral blood perfusion reduction associated with vertebral bone mineral density reduction: a dynamic contrast-enhanced MRI study in a rat orchiectomy model. J Magn Reson Imaging. 2008; 28:1515–1518.
13. Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012; 36:225–230.
14. Sanada M, Taguchi A, Higashi Y, Tsuda M, Kodama I, Yoshizumi M, et al. Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis. 2004; 176:387–392.
15. Sumino H, Ichikawa S, Kasama S, Takahashi T, Sakamoto H, Kumakura H, et al. Relationship between brachial arterial endothelial function and lumbar spine bone mineral density in postmenopausal women. Circ J. 2007; 71:1555–1559.
16. Williams JK, Honoré EK, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J Am Coll Cardiol. 1994; 24:1757–1761.
17. Wang YX, Zhou H, Griffith JF, Zhang YF, Yeung DK, Ahuja AT. An in vivo magnetic resonance imaging technique for measurement of rat lumbar vertebral body blood perfusion. Lab Anim. 2009; 43:261–265.
18. Zhang YF, Wang YX, Griffith JF, Kwong WK, Ma HT, Qin L, et al. Proximal femur bone marrow blood perfusion indices are reduced in hypertensive rats: a dynamic contrast-enhanced MRI study. J Magn Reson Imaging. 2009; 30:1139–1144.
19. Griffith JF, Yeung DK, Chow SK, Leung JC, Leung PC. Reproducibility of MR perfusion and (1)H spectroscopy of bone marrow. J Magn Reson Imaging. 2009; 29:1438–1442.
20. Pansini F, Bagni B, Bonaccorsi G, Albertazzi P, Zanotti L, Farina A, et al. Oophorectomy and Spine Bone Density: Evidence of a Higher Rate of Bone Loss in Surgical Compared with Spontaneous Menopause. Menopause. 1995; 2:109–115.
21. Prior JC, Vigna YM, Wark JD, Eyre DR, Lentle BC, Li DK, et al. Premenopausal ovariectomy-related bone loss: a randomized, double-blind, one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res. 1997; 12:1851–1863.
22. Griffith JF, Wang YX, Zhou H, Kwong WH, Wong WT, Sun YL, et al. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology. 2010; 254:739–746.
23. Basu S, Houseni M, Bural G, Chamroonat W, Udupa J, Mishra S, et al. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography: a novel application with significant implications for combined structure-function approach. Mol Imaging Biol. 2007; 9:361–365.
24. Chen WT, Shih TT, Chen RC, Lo SY, Chou CT, Lee JM, et al. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001; 220:213–218.
25. Kita K, Kawai K, Hirohata K. Changes in bone marrow blood flow with aging. J Orthop Res. 1987; 5:569–575.
26. Hartsock RJ, Smith EB, Petty CS. Normal variations with aging of the amount of hematopoietic tissue in bone marrow from the anterior iliac crest. A study made from 177 cases of sudden death examined by necropsy. Am J Clin Pathol. 1965; 43:326–331.
27. Ito H. Clinical considerations of regenerative medicine in osteoporosis. Curr Osteoporos Rep. 2014; 12:230–234.
28. Wang YX, Ng CK. The impact of quantitative imaging in medicine and surgery: charting our course for the future. Quant Imaging Med Surg. 2011; 1:1–3.
29. Masarapu V, Kim HL. Initial experience with arterial spin-labeling MR imaging to assess histology of renal masses. Quant Imaging Med Surg. 2013; 3:130–131.
30. Martirosian P, Boss A, Schraml C, Schwenzer NF, Graf H, Claussen CD, et al. Magnetic resonance perfusion imaging without contrast media. Eur J Nucl Med Mol Imaging. 2010; 37:Suppl 1. S52–S64.
31. Roldan-Valadez E, Piña-Jimenez C, Favila R, Rios C. Gender and age groups interactions in the quantification of bone marrow fat content in lumbar spine using 3T MR spectroscopy: a multivariate analysis of covariance (Mancova). Eur J Radiol. 2013; 82:e697–e702.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


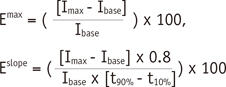





 XML Download
XML Download