Abstract
Objective
To describe the imaging features of pelvic solitary plasmacytoma and to correlate them with the pathologic grade.
Materials and Methods
A retrospective study was performed on the imaging features of 10 patients with a histological diagnosis of pelvic solitary plasmacytoma. The imaging studies were assessed for bone expansion, cortical destruction, signal intensity/density of soft tissue mass and enhancement manifestations, which were then correlated to the pathologic grade.
Results
The imaging features of pelvic solitary plasmacytoma revealed 3 different types: multilocular type (n = 5), unilocular type (n = 2) and complete osteolytic destruction type (n = 3) on computed tomography and MRI. Pathologically, the tumors were classified into low, intermediate and high grades. Features such as multilocular change, perilesional osteosclerosis, slight expansion, local bone cortex disruptions and masses inside bone destruction, often suggest a low-grade solitary plasmacytoma; complete osteolytic destruction, huge soft tissue mass, and osseous defects imply a higher pathologic grade.
Conclusion
Pelvic solitary plasmacytoma has various imaging manifestations, while a slight expansile osteolytic feature with multilocular change or homogeneous enhancement highly suggests its diagnosis. The distinctive imaging features of pelvic solitary plasmacytoma are well correlated to the pathologic grade.
Solitary plasmacytoma is characterized by a localized accumulation of neoplastic monoclonal plasma cells without proof of a systemic plasma cell proliferative disorder (1). It can be classified into 2 groups according to its location; solitary plasmacytoma of the bone and extramedullary plasmacytoma (2, 3). The axial skeleton is the most common location for the osseous lesions, while the upper respiratory tract is the most common location for extramedullary lesions. The male to female ratio is 2:1 and the median age at presentation is 55 years (4). However, preceding trauma may increase incidence of solitary plasmacytoma of the bone in younger people (5).
Solitary plasmacytoma has a significantly higher risk for progression to multiple myeloma. A moderate-dose radiotherapy combined with surgery is occasionally suggested for optimal treatment with sufficient local control. Nevertheless, for patients with high-grade histology, adjuvant chemotherapy may be considered (3). Holland et al. (6) showed that chemotherapy delays the progression time of plasmacytoma to multiple myeloma. The histologic grade is also linked to prognosis. The patient with low-grade extramedullary plasmacytoma reported recurring or developing myeloma at 120 months, while the high-grade tumor progressed at 26 months (7). Solitary plasmacytoma of the bone has a poorer prognosis than extramedullary plasmacytoma. The median time of progression to multiple myeloma following diagnosis was 25 and 45 months in solitary plasmacytoma of the bone and extramedullary plasmacytoma, respectively (8).
The radiologic findings of this disease have not been well documented thus far. We found only a small number of case reports on the radiological findings of pelvic solitary plasmacytoma and they mostly involved the sacrum (2, 9, 10, 11, 12). The imaging features with pathologic correlation have not been reported previously. Thus, the goal of the present study was to characterize the radiologic findings of pelvic solitary plasmacytoma and correlate those findings with its pathology.
We searched the pathology department database for cases of pelvic plasmacytoma diagnosed between December 2005 and March 2013. Thirty-four individuals were identified and chosen during this step. One author reviewed all the detailed medical record reports and imaging studies, including computed tomography (CT), magnetic resonance (MR), radionuclide bone imaging and 18F-fluorodeoxyglucose positron emission tomography. Twenty cases who presented with multiple myeloma at the time of diagnosis were excluded. Therefore, a total of 10 patients (6 males and 4 were females; age range, 35 to 75 years; mean age, 55.5 years) were analyzed in the study. The most frequent clinical signs and symptoms were skeletal pain (n = 8), pathologic fractures (n = 6) and local masses (n = 2); lymph node enlargement was not seen in this group of cases. The median follow-up period was 31.5 months (range, 2-82 months).
CT scanning was performed with a 16-slice multidetector CT scanner (Somatom Sensation, Siemens Medical Systems, Erlangen, Germany). All patients received a plain scanning. The scan parameters were 120 kV, 160 mA, with a slice thickness of 5 mm. Six cases had 90-100 mL of the nonionic contrast agent (iopamidol 300 mg L/mL) injected at an injection rate between 3.5-4.0 mL/s, for enhanced scanning. Images were obtained separately at the arterial phase (25-32 seconds after injection) and the venous phase (60-90 seconds after injection).
Eight of the 10 cases of pelvic solitary plasmacytoma (including 2 cases after radiotherapy) underwent MRI. MR scanning was performed using a 1.5-T or 3.0-T magnet (Signa, GE Medical Systems, Milwaukee, WI, USA) with an 8-channel torso-array coil. Axial T1-weighted images and T2-weighted images were obtained from 8 patients, and additional contrast-enhanced T1-weighted images (Omniscan, GE Healthcare, Princeton, NJ, USA, 0.2 mL/kg body weight) were obtained from all patients. The imaging parameters for T1-weighted images and T2-weighted images were as follows: repetition time/echo time of 440-550/7.4-8.6 ms and 2600-4000/80-126 ms. Additional diffusion weighted single-shot echo-planar imaging (DWI) was performed in 3 patients under breath-hold using the following parameters: 4000/72.3 ms, 6 mm thickness, matrix size = 128 × 128, b value = 0 and 700 s/mm2.
Two radiologists separately reviewed all CT and MR images and both were blinded to the identity of the patient and the clinical outcome. Any discordance was resolved by consensus. The images were then analyzed for the following features: bone expansion, cortical destruction, signal intensity/density of soft tissue mass and enhancement manifestations.
The pathologic specimens were obtained from the 10 patients with pelvic solitary plasmacytoma for pathologic correlation. The histological diagnosis of solitary plasmacytoma of the bone was based on the World Health Organization classification system for hematologic malignancies: it requires a single area of bone damage due to clonal plasma cell hyperplasia; histologically normal marrow aspirate and trephine samples; normal skeletal survey results; no anemia, hypercalcemia, or renal impairment attributable to plasma cell dyscrasia; little or no serum or urinary monoclonal immunoglobulin (level of 0.20 g/L, possibly indicative of multiple myeloma); and no additional lesions visible upon MR scan of the spine 9 (3, 13, 14). Cases were graded according to the histological grading criteria devised by Bartl et al. (15) for multiple myeloma. This involves a 3-tiered grading system summarized as follows:
Grade I (low grade), comprised of the Marschalko and small cell types. Figure 1A showed a Marschalko type in which the plasma cells were indistinguishable from normal cells, although mitotic figures were seen.
Grade II (intermediate grade), consisted of the cleaved, polymorphous, and asynchronous cell types. Figure 2A showed the asynchronous type in which there is marked discrepancy of maturation between the nucleus and cytoplasm. At least 50% of the cells had enlarged nuclei with prominent nucleoli, while the abundant basophilic cytoplasm and perinuclear hof were maintained.
Grade III (high grade), represented the plasmablastic type. Figure 3A showed a plasmablastic type with large nuclei and very prominent centrally located nucleoli. Cytoplasm was confined to a fairly narrow rim. Perinuclear hof was inconspicuous or absent.
The correlation between the imaging findings and the pathologic features was assessed by a radiologist who reviewed the CT images, and by a pathologist who reviewed the pathologic reports and the histological slices. Pathologic reviews were made while paying particular attention to specific features in the solitary plasmacytoma lesions.
All 10 cases of pelvic solitary plasmacytoma revealed 3 different CT findings:
1) The multilocular type: 5 cases, with 2 cases in the sacrum and the ilium respectively, and 1 case in the ischium. The 2 cases in the sacrum showed a slight expansile osteolytic lesion with multilocular change, consisting of the mass itself and the remaining sacrum. The 2 cases in the ilium showed slight expansion, partial multilocular change (Fig. 2B, C). The case involving the ischium showed expansile osteolytic lesion with bone cortex disruptions. Three of the 5 cases with contrast-enhanced CT scanning, showed marked homogeneous enhancement.
2) The unilocular type: 2 cases in the sacrum, showed slight expansile osteolytic lesion with marked homogeneous enhancement in both cases and perilesional osteosclerosis in 1 case (Fig. 1B, C).
3) The complete osteolytic destruction type: 3 cases in the ilium, showed slight expansion, bone cortex disruptions, osseous defects, and a large soft tissue mass extending outside the bone structure. One of the 3 cases with contrast-enhanced CT scanning, showed marked homogeneous enhancement.
MRI in 8 patients revealed the following:
1) Seven cases before radiotherapy showed low signal intensity lesions on T1-weighted images, with high signal intensity lesions on T2-weighted images in 5 of the 7 cases (Fig. 4A). The other 2 cases showed heterogeneous low signal intensity lesions on T1-weighted images and heterogeneous high signal intensity on T2-weighted images; high signal intensity lesions on DWI (Fig. 4B) in 3 cases; homogeneous enhancement in 5 cases and heterogeneous enhancement in 2 cases (Fig. 3B).
2) Two cases after radiotherapy showed low signal intensity on both T1-weighted images and T2-weighted images (Fig. 5); and heterogeneous enhancement on meglumine gadoterate-enhanced T1-weighted image.
All tumors were consistent with a diagnosis of solitary plasmacytoma of the bone, with 3 cases in Grade I, 4 in Grade II, and 3 in Grade III.
The CT findings correlated with the pathologic grades and revealed the following: 1) Grade I showed slight expansion, bone cortex thinning and disruption, complete multilocular change or unilocular change with perilesional osteosclerosis and lesions with marked homogeneous enhancement. 2) Grade II showed partial multilocular change or unilocular change without osteosclerosis, bone cortex disruptions and masses inside the bone structure with marked homogeneous enhancement. 3) Grade III showed complete osteolytic destruction, bone cortex disruptions and osseous defects, and a large soft tissue mass outside the bone with homogeneous heterogeneous enhancement.
MRI findings of the pelvic solitary plasmacytoma (7 cases before radiotherapy) correlated with the pathologic features revealed the following: 1) Grade I showed well-defined lesions with marked homogeneous enhancement, the remaining bone inside or around the lesion showed linear low signal inside on T2-weighted images and DWI. 2) Grade II showed masses of homogeneous signal intensity inside the bone structure, less remaining viable bone, homogeneous enhancement and high signal on DWI. 3) Grade III showed huge masses extending outside the bone structure with homogeneous (1/3) or heterogeneous (2/3) signal intensities.
The imaging features and the pathologic findings were summarized in Table 1.
One of 3 cases with Grade I pathology, had a recurrence of low-grade tumor at 36 months after diagnosis and subsequently developed disseminated bone disease of myeloma at 38 months. Two of the 4 cases progressed to multiple myeloma at 25 and 33 months after diagnosis, respectively. One of the 3 cases in Grade III developed recurrence at 12 months.
Imaging reports have indicated that solitary plasmacytoma of the bone are mostly located in the vertebra and long bone (16, 17). The number of publications on the imaging of pelvic solitary plasmacytoma is very limited. The published radiological findings of solitary plasmacytoma of the bone were mostly an expansile osteolytic lesion with marked enhancement on CT or MRI. Kosaka et al. (9) reported that 3 cases of sacral solitary plasmacytoma showed relatively low signal intensity on T2-weighted images. Our study provided a somewhat different but interesting insight on the subject.
The CT features of pelvic solitary plasmacytoma can be summarized as 3 types: multilocular type, unilocular type and complete osteolytic destruction type. The multilocular type includes complete multilocular change and partial multilocular change. The former is seen in the sacrum, consisting of the mass itself and the remaining sacrum; the lesion consists of the expansion and destruction of the bone cortex, sometimes with a thinning bone ridging around the periphery, creating a "soap bubble" appearance. The latter is seen in the ilium or the ischium, similar to the "mini brain" described in a spinal solitary plasmacytoma (16). This multilocular change has not been described for pelvic solitary plasmacytoma in previous literature, nor has it ever been seen in other tumors like osteosarcoma, lymphoma and chordoma, during our long-term clinical work. Therefore, we believe that this change has particular importance for the diagnosis of pelvic solitary plasmacytoma. The unilocular type is seen in the sacrum and is compatible with the previous literature (9). This type demonstrates slight expansion with soft tissue mass showing marked homogeneous enhancement on the enhanced CT. The complete osteolytic destruction type is seen in the ilium, showing slight expansion but with huge soft tissue mass extending outside the bone structure, which in fact is not compatible with findings in the previous literature. We believe that this manifestation may be related to their higher degree of malignancy.
MRI is less advantageous compared to CT in displaying the imaging features of bone destruction, like multilocular or unilocular change, expansion lesions or punched-out defects. It can only demonstrate some linear low signal intensity inside the soft tissue mass or the dark signal bone cortex around the mass. Nevertheless, MRI still has its particular imaging features and specific values, which makes it indispensable to imaging technology. The solitary plasmacytoma of the bone lesions before treatment often show homogeneous low signal intensity on T1-weighted images and high signal on T2-weighted images. None of our cases showed low signal intensity on T2-weighted images, which was contrary to published findings (9). However, MRI is more advantageous than CT in displaying the enhancement features of the mass. The marked homogeneous enhancement on MR images may be helpful for the diagnosis of pelvic solitary plasmacytoma. Furthermore, DWI of pelvic solitary plasmacytoma has not been reported previously. We found that the pelvic solitary plasmacytoma masses showed high signal on DWI. We believe that this feature is helpful for diagnosis; furthermore, it may be triggered by plasma cell aggregation, causing limited diffusion. Given the low number of cases on DWI, further studies are clearly needed. Moreover, MRI is more suitable for reexamination after radiotherapy to show a curative effect that includes size and signal change. Two cases after radiotherapy in our groups showed low signal intensity on T2-weighted images and heterogeneous enhancement on MRI. We inferred that the low signal intensity on T2-weighted images might be related to post-radiotherapy fibrous tissue hyperplasia, while the heterogeneous enhancement may have resulted from necrosis or degeneration within the masses (18).
The relationship between imaging features and pathological grading of pelvic solitary plasmacytoma has not been reported previously. We found that distinctive CT imaging appearances of pelvic solitary plasmacytoma are closely correlated to its pathologic grades. Features such as multilocular change, slight expansion, local bone cortex disruptions, perilesional osteosclerosis, masses inside bone destruction, often suggest a low-grade solitary plasmacytoma of the bone. A more obvious and distinct multilocular change indicates a lower pathologic grade. While advanced solitary plasmacytoma of the bone often has complete osteolytic destruction with little or no local multilocular change, there are also large soft tissue masses, obvious bone cortex disruptions, and osseous defects. However, the enhancement degree of the mass may not be related to its pathologic grades.
The MR imaging findings of pelvic solitary plasmacytoma also correlated to its pathologic grades, especially to the manifestation of soft tissue mass. The mass is often confused with a low pathologic grade, and vice versa, since it is similar to primary bone formation or within the bone destruction, and has homogeneous or some linear low signal intensity within, or perilesional dark rim (remaining bone). The signal intensity on both T1-weighted images and T2-weighted images is thought to be unrelated to the pathologic grades.
Imaging studies showed that pelvic solitary plasmacytoma has obvious malignant tumor characteristics, and therefore pelvic solitary plasmacytoma should be considered in the differential diagnosis of many primary bone tumors and metastasis. The main differential diagnosis would be lymphoma based on its imaging features, such as slight bone destruction and homogeneous enhancement. Bone destruction causing multilocular change has not been reported previously in lymphomatous lesions. The signal intensity on T2-weighted images was lower than solitary plasmacytoma (19). The differential diagnosis should include chordoma, which is also low-grade malignant tumor, in cases of sacral solitary plasmacytoma. The median age of incidence of chordoma is slightly higher than solitary plasmacytoma, and the imaging of chordoma often show complete bone destruction, sometimes with remaining bone, but seldom causes multilocular change (20).
In conclusion, pelvic solitary plasmacytoma often presents with a slight expansile osteolytic lesion, especially a lesion with multilocular change or a mass with homogeneous enhancement, and also a lesion showing high signal on DWI. The distinctive image appearance of pelvic solitary plasmacytoma closely correlated to its pathologic grades and may be of value in judging prognosis. CT is superior to MRI in displaying bone destruction and its correlated pathologic grades, while MRI is suitable for the reexamination after radiotherapy.
Figures and Tables
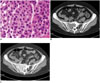 | Fig. 1Unilocular type solitary plasmacytoma in 58-year-old female (case 6).
A. Pathologic hematoxylin and eosin staining (× 400) demonstrates low-grade plasmacytoma with plasma cells indistinguishable from normal cells. B. Unenhanced CT shows slight expansile osteolytic lesion with perilesional osteosclerosis. C. Contrast-enhanced CT shows lesion with marked homogeneous enhancement.
|
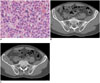 | Fig. 2Multilocular type solitary plasmacytoma in 52-year-old man (case 3).
A. Pathologic hematoxylin and eosin staining (× 400) demonstrates intermediate-grade plasmacytoma with plasma cells containing large eccentric nuclei with prominent nucleoli and abundant basophilic cytoplasm. B, C. Bone window CT shows slight expansile osteolytic lesion with partial multilocular change (B) and local bone cortex interruption (C) in right ilium.
|
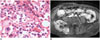 | Fig. 3Complete osteolytic destruction type solitary plasmacytoma in 75-year-old female (case 9).
A. Pathologic hematoxylin and eosin staining (× 400) demonstrates high-grade plasmacytoma with many cells exhibiting plasmablastic features with frequent mitoses. B. Enhanced T1-weighted image in axial plane shows heterogeneous enhancement of soft tissue mass in right ilium.
|
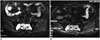 | Fig. 4Multilocular type solitary plasmacytoma in 57-year-old female (case 2).
A, B. Axial fat suppressed T2-weighted sequences (A) and diffusion-weighted imaging (B) shows high signal with linear low signal within lesion in sacrum.
|
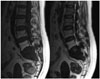 | Fig. 5Multilocular type solitary plasmacytoma in 56-year-old man (case 1). T1-weighted images and T2-weighted images in sagittal plane show low signal of lesions and high signal of fifth lumbar vertebra after radiotherapy. |
Table 1
Summary of Imaging Features and Pathologic Grades in 10 Cases

Acknowledgments
The authors thank the Pathology and Radiology Department staff members of the Second Affiliated Hospital of Zhejiang University School of Medicine for providing patient information and giving valuable comments and advice for the study.
References
1. Ellis PA, Colls BM. Solitary plasmacytoma of bone: clinical features, treatment and survival. Hematol Oncol. 1992; 10:207–211.
2. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. ScientificWorldJournal. 2012; 2012:895765.
3. Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol). 2004; 16:405–413.
4. Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000; 96:2037–2044.
5. Pasch W, Zhao X, Rezk SA. Solitary plasmacytoma of the bone involving young individuals, is there a role for preceding trauma. Int J Clin Exp Pathol. 2012; 5:463–467.
6. Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992; 69:1513–1517.
7. Susnerwala SS, Shanks JH, Banerjee SS, Scarffe JH, Farrington WT, Slevin NJ. Extramedullary plasmacytoma of the head and neck region: clinicopathological correlation in 25 cases. Br J Cancer. 1997; 75:921–927.
8. Suh YG, Suh CO, Kim JS, Kim SJ, Pyun HO, Cho J. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol. 2012; 91:1785–1793.
9. Kosaka N, Maeda M, Uematsu H, Matsumine A, Koshimoto Y, Itoh H. Solitary plasmacytoma of the sacrum. Radiologic findings of three cases. Clin Imaging. 2005; 29:426–429.
10. Wong CL, Mansberg R. Solitary plasmacytoma of bone: an unusual cause of severe sacral pain in a young man. Clin Nucl Med. 2005; 30:612–614.
11. Llauger J, Palmer J, Amores S, Bagué S, Camins A. Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol. 2000; 174:417–424.
12. Diel J, Ortiz O, Losada RA, Price DB, Hayt MW, Katz DS. The sacrum: pathologic spectrum, multimodality imaging, and subspecialty approach. Radiographics. 2001; 21:83–104.
13. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–87.
14. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London: British Committee for Standards in Haematology;Accessed October 14, 2013. Web site. http://www.guideline.gov/content.aspx?id=15514.
15. Bartl R, Frisch B, Fateh-Moghadam A, Kettner G, Jaeger K, Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987; 87:342–355.
16. Major NM, Helms CA, Richardson WJ. The "mini brain": plasmacytoma in a vertebral body on MR imaging. AJR Am J Roentgenol. 2000; 175:261–263.
17. Ooi GC, Chim JC, Au WY, Khong PL. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am J Roentgenol. 2006; 186:821–827.
18. Glazer HS, Niemeyer JH, Balfe DM, Hayden RE, Emami B, Devineni VR, et al. Neck neoplasms: MR imaging. Part II. Posttreatment evaluation. Radiology. 1986; 160:349–354.
19. Kirsch J, Ilaslan H, Bauer TW, Sundaram M. The incidence of imaging findings, and the distribution of skeletal lymphoma in a consecutive patient population seen over 5 years. Skeletal Radiol. 2006; 35:590–594.
20. Taki S, Kakuda K, Kakuma K, Yamashita R, Kosugi M, Annen Y. Posterior mediastinal chordoma: MR imaging findings. AJR Am J Roentgenol. 1996; 166:26–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download