Abstract
Thyroid cancer may have small adipose structures detected by microscopy. However, there are no reports of thyroid cancer with gross fat evaluated by radiological methods. We reported a case of a 58-year-old woman with a fat containing thyroid mass. The mass was hyperechoic and ovoid in shape with a smooth margin on ultrasonography. On computed tomography, the mass had markedly low attenuation suggestive of fat, and fine reticular and thick septa-like structures. The patient underwent a right lobectomy. The mass was finally diagnosed as a follicular variant of papillary thyroid cancer with massive stromal fat.
Fat-containing lesions in the thyroid are very rare (1). Thyroid lesions containing gross fat include heterotopic fat nests, thyrolipoma, teratoma, liposarcoma, and thyroid cancers of the papillary or follicular type (234567). Most of these cases are only described upon obtaining pathological data after surgery; radiologic methods are of limited use (89). With the approval of the Institutional Review Board of our hospital, we reported the case of an intrathyroid nodule composed mainly of gross fat detected by computed tomography (CT) and confirmed by lobectomy as a follicular variant of papillary thyroid cancer (FVPTC) with mature fat.
A 58-year-old woman presented with an incidentally detected mass in the thyroid on routine ultrasonography (US) at a primary clinic. The patient had a 10-year history of diabetes mellitus and hypertension, and was currently taking medication. She had no medical history of thyroid disease. Physical examination revealed a firm mass of approximately 3 cm in size in the right anterior neck. Laboratory tests revealed that serum anti-microsomal antibody, anti-thyroglobulin antibody, thyroglobulin, calcitonin, T3, free T4, and thyroid-stimulating hormone levels were within normal ranges.
Ultrasonography revealed an ovoid lesion with a smooth margin of approximately 2.9 × 2 × 1.3 cm in size. The nodule was generally hyperechoic, with the peripheral portion more hyperechoic than the central area. Posterior to the mass, curtain-like hyperechoic shadowing was observed. The perithyroidal fat, muscle, and vertebral bodies were therefore not clearly visualized (Fig. 1A). Doppler US did not show any vascular signals in the nodule. Based on these findings, a diagnosis of an indeterminate nodule was made. A board-certified radiologist performed US-guided fine needle aspiration using a freehand technique with a 23-gauge needle and a 5-mL disposable plastic syringe. Cytologic smears were made on glass slides and immediately fixed in 95% alcohol for both Papanicolaou staining and May-Grunwald-Giemsa staining. Cytology revealed atypia of undetermined significance or a follicular lesion of undetermined significance with negative for BRAF mutations and positive for an NRAS 61 mutation.
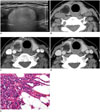
The thyroid CT protocol at our hospital includes pre-contrast, early, and delayed phases. Pre-contrast images were acquired from the mandibular angle to the thoracic inlet. Early phase images (40 seconds) were scanned from the hyoid bone to the thoracic inlet to cover the entire thyroid and level III-VI lymph nodes. Delayed scans (90 seconds) covered the area from the skull base to the upper mediastinum. All scans were acquired with a slice thickness of 2.5 mm and reconstruction increment of 2.5 mm. Pre-contrast CT showed markedly low attenuation (mean CT number, -80 Hounsfield units [HU]), in a well-defined mass with fine reticular and thick septa-like structures in the parenchyma of the right mid-to-upper portion of the thyroid (Fig. 1B). To measure the enhancement pattern of the tumor, 25 mm2 of the region of interest was placed in the area of the fatty mass, including the reticular and thick septa-like soft tissue lesions. The tumor was enhanced in the early phase (mean CT number, 16 HU) and was washed out in the delayed phase (mean CT number, -13 HU) (Fig. 1C, D). The tumor was ovoid in the longitudinal direction, had a lobular appearance in the axial plane, and was completely encapsulated in the thyroid parenchyma, with no evidence of extrathyroidal extension. No pathological lymph nodes were identified. Based on these findings, we suspected a fat-containing, neoplastic lesion of the thyroid gland, such as thyrolipoma, teratoma, lipoma, low-grade liposarcoma, or a follicular tumor with stromal fat. Considering the results from cytopathology and the gene mutation analysis, we could not rule out the possibility of a malignant lesion. Therefore, we performed a right lobectomy.
The gross specimen was fixed with formalin solution. It showed a yellowish, homogeneous, and well-demarcated nodular lesion measuring 2.5 × 2.2 × 1.5 cm at the upper-to-mid pole. The mass was 1.5 cm away from the isthmic resection margin.
Microscopic examination revealed a well-circumscribed, thin, fibrous, and capsulated nodule with mature fat. There were atypical follicular cells with nuclear enlargement, nuclear overlapping, chromatin clearing, and nuclear grooves without papillary structures in the nodule (Fig. 1E). On immunohistochemistry, the tumor cells stained positive for HBME-1 (DAKO, Glostrup, Denmark, 1:100) and galectin-3 (Novocastra, Newcastle Upon Tyne, UK, 1:200). Finally, we tested for the V600E and K601E mutations in exon 15 of the BRAF gene and codons 12, 13, and 61 of the KRAS and NRAS genes. We reconfirmed positive NRAS 61 mutation, negative BRAF and negative KRAS mutation. The final pathologic diagnosis was follicular variant papillary thyroid carcinoma with mature fat.
In our case, the tumor was a yellowish, homogeneous, and well-demarcated nodular lesion on gross examination, with negative value of attenuation on CT, suggesting fat. However, it was difficult to recognize the tumor as a fat containing mass on US examination. The hyperechoic nature of the mass and the curtain-like hyperechoic shadowing posterior to the mass may suggest rich fatty component. We suspected that the hyperechoic shadowing might have been due to a reverberating artifact caused by innumerable fat globules and septa of the tumor, similar to the artifact frequently observed along the thick abdominal walls that consist of several layers of fat globules surrounded by connective tissues, skin, and fascia.
Computed tomography and gross specimen findings of our case were very similar to those of thyrolipoma, a benign fat-containing tumor (810). Thyrolipoma is considered a variant of follicular adenoma, which is characterized by a well-circumscribed and encapsulated nodule resulting from the proliferation of thyroid follicles admixed with mature fat (1011). This case could have been misdiagnosed as a follicular neoplasm or thyrolipoma, as the tumor cells did not show a papillary growth pattern upon microscopic examination and the gross features were very similar to thyrolipoma. Two reports provided the CT and magnetic resonance imaging findings of thyrolipoma (812). The mass predominantly had fat attenuation and distinct margins in the thyroid gland. However, the authors only performed pre-contrast CT on the lesion. Therefore, it is uncertain whether thyrolipoma and thyroid cancer with massive stromal fat can be differentiated by CT with contrast enhancement. In our case, the tumor was enhanced in the early phase and slightly washed out in the delayed phase.
Fat-containing thyroid cancer of the papillary or follicular type, have only been reported from pathological studies (2456). Fat tissue was observed only by microscopy, so it was assumed that small amounts of fat tissue could not be detected by CT. We also assumed that the detectability of the fat component is variable, as it depends on the total number of fat cells in the mass. Mature teratoma can be recognized if the fat-containing mass contains calcification, bone or cartilage, and cystic components. However, this is not possible, as radiological images do not always exhibit all these attributes (7). CT images of liposarcoma (3) in the thyroid gland are very similar to those in our case, and differentiating between teratoma, thyrolipoma, liposarcoma, and papillary thyroid cancer with massive fat may be impossible using radiology alone.
The origins of the fat tissue are unknown. Some investigators have insisted that fat cells are derived from displaced remnants of embryonic structures in the thyroid. In amyloid goiter, Schröder and Böcker (1) postulated that adipose tissue may be derived from metaplasia of stromal fibroblasts due to either impaired circulation and diffusion or tissue hypoxia caused by amyloid deposition. DeRienzo and Truong (5) suggested that the fat cells in thyrolipoma and follicular carcinoma might be neoplastic tissue because 1) fat did not exist in the normal thyroid, 2) fat was a major component of the tumor, 3) there was no evidence of degenerative changes such as dystrophic calcification, cystic or hemorrhagic degeneration, or amyloid deposition, 4) fat was widely distributed in the tumor, and 5) there were similar fat-containing tumors in other organs such as thymolipoma, parathyroid adenoma, and fat-containing fibroadenoma of the breast. Our case showed similar microscopic features to theirs in agreement with the suggestion that the fat cells might be neoplastic cells themselves.
In summary, we identified FVPTC with stromal fat using US and CT. The diagnosis was confirmed by histopathology, immunochemical staining, and gene analysis. These findings included a hyperechoic mass with a smooth margin with a reverberating artifact and no detectable Doppler signal on US. CT revealed a well-encapsulated fat-attenuation mass with enhancing septa-like structures. FVPTC should be included in the differential diagnosis of fat-containing focal lesions in the thyroid, in addition to thyrolipoma, teratoma, liposarcoma, and small focal fat nests near the capsule. Moreover, fine needle aspiration cytology with gene analysis may be helpful in determining the nature of fatty tumors before surgery.
Figures and Tables
Fig. 1
58-year-old woman diagnosed with follicular variant of papillary thyroid cancer with mature fat.
A. Longitudinal image of right thyroid using gray-scale ultrasonography shows hyperechoic, ovoid mass with smooth margin. Peripheral portion of mass is more echogenic than central area. Curtain-like hyperechoic shadowing was observed posterior to mass. Perithyroidal fat, muscle, and vertebral bodies were therefore not clearly visualized. B-D. Pre-contrast computed tomography (CT) shows fatty (mean CT number, -80 Hounsfield units, HU), well-defined mass in parenchyma of right mid-to-upper portion of thyroid (B). Mass has several fine reticular and thick septa-like soft tissue lesions, which show contrast enhancement. Post-contrast CT shows higher enhancement in early phase (C, at 40 seconds; mean CT number, 16 HU) of tumor than in delayed phase (D, 90 seconds; mean CT number, -13 HU). Tumor is lobular in appearance in axial plane (B-D), ovoid in longitudinal direction, and completely located in thyroid parenchyma without evidence of extrathyroidal extension. E. High-power magnification (1:400, hematoxylin and eosin staining) reveals tumor cells with enlarged nuclei, chromatin clearing, and nuclear grooves. Tumor cells are surrounded by mature adipose tissue.
References
1. Schröder S, Böcker W. Lipomatous lesions of the thyroid gland: a review. Appl Pathol. 1985; 3:140–149.
2. Akslen LA, Maehle BO. Papillary thyroid carcinoma with lipomatous stroma. Am J Surg Pathol. 1997; 21:1256–1257.
3. Azar AR, Weynand B, Daumerie C, Coche E. Metastatic liposarcoma of the thyroid gland. Br J Radiol. 2003; 76:750–752.
4. Bisi H, Longatto Filho A, de Camargo RY, Fernandes VS. Thyroid papillary carcinoma lipomatous type: report of two cases. Pathologica. 1993; 85:761–764.
5. DeRienzo D, Truong L. Thyroid neoplasms containing mature fat: a report of two cases and review of the literature. Mod Pathol. 1989; 2:506–510.
6. Vestfrid MA. Papillary carcinoma of the thyroid gland with lipomatous stroma: report of a peculiar histological type of thyroid tumour. Histopathology. 1986; 10:97–100.
7. Zhang YZ, Li WH, Zhu MJ, Li YH, Gao Y. An unusual mature thyroid teratoma on CT and 99Tcm scintigraphy imaging in a child. Pediatr Radiol. 2010; 40:1831–1833.
8. Demirpolat G, Guney B, Savas R, Tuncay G, Alper H, Sener RN. Radiologic and cytologic findings in a case of thyrolipoma. AJNR Am J Neuroradiol. 2002; 23:1640–1641.
9. Kim KH, Seo HS, Lee YH, Lee KY, Kim YS, Son GS, et al. Study of intrathyroid fat-containing lesions using CT imaging with literature review. Neuroradiology. 2013; 55:1405–1411.
10. Ge Y, Luna MA, Cowan DF, Truong LD, Ayala AG. Thyrolipoma and thyrolipomatosis: 5 case reports and historical review of the literature. Ann Diagn Pathol. 2009; 13:384–389.
11. Daboin KP, Ochoa-Perez V, Luna MA. Adenolipomas of the head and neck: analysis of 6 cases. Ann Diagn Pathol. 2006; 10:72–76.
12. Borges A, Catarino A. Case 53: adenolipoma of the thyroid gland. Radiology. 2002; 225:746–750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download