Abstract
Objective
The purpose of this study was to evaluate the reliability of a new magnetic resonance imaging (MRI) grading system for cervical neural foraminal stenosis (NFS).
Materials and Methods
Cervical NFS at bilateral C4/5, C5/6, and C6/7 was classified into the following three grades based on the T2-weighted axial images: Grade 0 = absence of NFS, with the narrowest width of the neural foramen greater than the width of the extraforaminal nerve root (EFNR); Grade 1 = the narrowest width of the neural foramen the same or less than (but more than 50% of) the width of the EFNR; Grade 2 = the width of the neural foramen the same or less than 50% of the width of the EFNR. The MRIs of 96 patients who were over 60 years old (M:F = 50:46; mean age 68.4 years; range 61-86 years) were independently analyzed by seven radiologists. Interobserver and intraobserver agreements were analyzed using the percentage agreement, kappa statistics, and intraclass correlation coefficient (ICC).
Results
For the distinction among the three individual grades at all six neural foramina, the ICC ranged from 0.68 to 0.73, indicating fair to good reproducibility. The percentage agreement ranged from 60.2% to 70.6%, and the kappa values (κ = 0.50-0.58) indicated fair to moderate agreement. The percentages of intraobserver agreement ranged from 85.4% to 93.8% (κ = 0.80-0.92), indicating near perfect agreement.
Cervical neural foraminal stenosis (NFS) is defined by neural foraminal narrowing that may be caused by degenerative osteophytes, facet hypertrophy, or laterally herniated discs (123). The progressive narrowing of the intervertebral foramina by these anatomical changes may result in nerve root impingement, inflammation, or both, which could cause cervical radiculopathy.
Magnetic resonance imaging (MRI) is currently the most commonly used imaging method for the accurate evaluation of the cervical spine. MRI can demonstrate the cervical spinal anatomy, including the bony structure, spinal cord, and soft tissue, but assessing cervical foraminal stenosis with MRI is demanding because of the small size and susceptibility of the anatomical parts of the cervical spine (456). A number of reports have assessed cervical NFS (278910111213). However, to the best of our knowledge, there is no universally accepted grading system for cervical NFS based on MRI.
A standardized grading system for assessing cervical NFS is required for comparing the data from different investigations and for clinical research and is helpful for consistent radiology reporting, identifying the correlation between the imaging findings and clinical symptoms during daily routine practice, improving communication between radiologists and clinicians, and the standardized treatment protocol of cervical radiculopathy. Therefore, we developed a new MRI grading system for diagnosing and grading cervical NFS based on axial T2-weighted images.
The purpose of this study was to propose a new MRI grading system for cervical NFS and to evaluate the reproducibility of the system.
Cervical NFS was classified into one of the following three grades by the MR findings on routinely obtained axial T2-weighted images at the cervical disc level (Fig. 1). Grade 0 refers to the absence of NFS, with the narrowest width of the neural foramen greater than the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process (Fig. 2). Grade 1 refers to moderate cervical NFS: the narrowest width of the neural foramen is 51-100% of the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process (Fig. 3). Grade 2 refers to severe cervical NFS, when the width of the neural foramen is the same as or less than 50% of the width of the extraforaminal nerve roots (Fig. 3). The cases that showed nearly complete obliteration of the neural foramen were classified as Grade 2 regardless of the extraforaminal nerve root. In cases of an unclear ipsilateral extraforaminal nerve root on MR axial images, the width of the contralateral extraforaminal nerve root at the level of the superior articular process or the width between the posterior margin of the vertebral artery and the anterior margin of the superior articular process were alternatively used. Because the vertebral arteries are located in the transverse foramen of each cervical vertebra, the posterior margin of the vertebral artery was used as an alternative standard reference for the anterior margin of the extraforaminal nerve root space for the grading system. In addition, the anterior margin of the superior articular process was used as a standard reference for the posterior margin of the extraforaminal nerve root space because of the low prevalence of anatomical variation.
This study was approved by our Institutional Review Board, and informed consent was waived. Cervical NFS is known to be common in older age groups because degenerative changes in the cervical spine are the most common cause of cervical NFS and degenerative changes increase with increasing age (141516). This retrospective study involved cervical spinal MRIs of 96 patients aged over 60 years. Patients with either history of trauma, previous spinal operation, or histologically proven or suspected tumor were excluded on the basis of medical records reviewed by one of the authors. Sixty-six patients who had undergone cervical spinal MR images in our institution between the period of January and December 2010 were consecutively selected. To include MR images from various scanners, another 30 patients with cervical spinal MR images that had been imported from outside hospitals were also consecutively selected. As a result, 96 patients were included in the final analysis (male:female = 50:46; mean age, 68.4 years; range, 61-86 years).
At our institution, the MR images were acquired with either a 1.5T scanner or 3T scanner (Gyroscan Intera Achieva; Philips Medical Systems, Amsterdam, the Netherlands) using a 16 channel neurovascular coil for the 3T imager or head and neck coil for the 1.5T imager, with the patient in the supine position. Axial T2-weighted images at the cervical disc level were usually acquired parallel to the disc spaces with the following parameters: repetition time 3131-4307 ms; echo time 80-120 ms; field of view = 150-150 mm; section thickness = 3 mm; number of axial sections in each disc = 4.
The imported MR images that were scanned at diverse hospitals before referral to our hospital were taken with various MR scanners using different protocols. Axial T2-weighted images (n = 30) were selected for grading. All of the images were de-identified before the analysis and were randomly numbered from 1 to 96.
Seven observers from different institutions and with different levels of experience-two radiology residents and five musculoskeletal radiologists with 2, 4, 7, and 17 years of experience-independently interpreted the images, blinded to the patient's history and age. The images were given in the form of a presentation slide as a set of six images for each case, two axial plane images of each of three sequential disc levels (C4/5, C5/6, and C6/7), along with the image adjacent to the disc plane, and these were selected to show the bilateral neural foramina. The grading system was introduced to the readers as a written description with diagrammatic examples only (Fig. 1); no detailed explanation was given. The observers were asked to rate the degree of cervical NFS according to the aforementioned grading system at the bilateral sides of the three sequential levels (C4/5 to C6/7). The upper cervical levels were excluded because of the low prevalence of NFS (1617). To assess the intraobserver variability, one reader interpreted the images twice at the interval of six months in order to minimize observer memory bias. To evaluate the assessment of extraforaminal nerve root visibility, two readers interpreted the visibility of the extraforaminal nerve root in consensus. The frequency of unclear extraforaminal nerve roots on MR axial images was as follows: 2.1% (n = 2) at the right side C4/5 level; 2.1% (n = 2) at the left side C4/5 level; 1.0% (n = 1) at the right C5/6 level; 2.1% (n = 2) at the left C5/6 level; 2.1% (n = 2) at the right C6/7 level; 2.1% (n = 2) at the left C6/7 level; and 1.0% (n = 1) at bilateral C6/7 level. The width of the contralateral extraforaminal nerve root was used as a reference in most of these cases (n = 11).
Percentage agreement, kappa statistics, and intraclass correlation coefficient (ICC) with two-way random effects were used to evaluate the intraobserver and interobserver reliability of the new MR grading system for 1) the distinction among the three individual grades, 2) the distinction between non-severe and severe stenosis (Grades 0 and 1 vs. Grade 2), and 3) the presence of NFS (Grade 0 vs. Grades 1 and 2). Regarding the distinction among the three individual grades, agreement was measured using the linear weighted Cohen kappa statistic, giving greater weight to a difference of more than one step between observers than to a difference of only one step (18).
The percentage agreement and kappa statistic were assessed for all 21 reader pairs, and the result was averaged (1920). The overall percentage agreements were also calculated. Agreement was rated as follows: kappa value interpretations were poor (κ < 0.1), slight (0.1 ≤ κ ≤ 0.2), fair (0.2 < κ ≤ 0.4), moderate (0.4 < κ ≤ 0.6), substantial (0.6 < κ ≤ 0.8), and nearly perfect (0.8 < κ ≤ 1.0) (1921). Among the various guidelines for interpreting ICCs, we adapted a scale that was introduced in previous literature: ICC values of less than 0.40 indicate poor reproducibility, values of 0.40-0.75 indicate fair to good reproducibility, and values greater than 0.75 indicate excellent reproducibility (2022). The Statistical Package for the Social Sciences for Windows (SPSS Statistics for Windows Version 17.0; SPSS Inc., Chicago, IL, USA) and Medcalc software (version 11.1.1.0 for Microsoft Windows 2000/XP/Vista/7; Medical Software, Mariakerke, Belgium) were used for the statistical analyses.
The distribution of the overall stenoses is shown on Table 1. Grade 0 (no stenosis) was most frequently noted, and the overall burden of stenosis was greatest at the C6/7 level.
Table 2 shows the interobserver agreements of all readers, experienced readers, and inexperienced readers with respect to percentage agreement, kappa statistics, and ICC.
For the distinction among the three individual grades at all six neural foramina, the ICC ranged from 0.68 to 0.73, indicating fair to good reproducibility. The percentage agreement ranged from 60.2% to 70.6%, and the kappa values (κ = 0.50-0.58) indicated fair to moderate agreement. A slightly higher level of agreement was seen for the presence of cervical NFS (Grade 0 vs. Grades 1 and 2), specifically, 75.4% to 83.8%; κ = 0.51-0.64. Agreement was slightly higher for the classification of cases as either non-severe (Grades 0 and 1) or severe (Grade 2) stenosis; the percentage agreement ranged from 74.4% to 90.2%, and the kappa values were 0.47-0.53.
The average percentage agreements ranged from 65.1% to 86.6% (κ = 0.50-0.52) at the right side C4/5 level; 70.6% to 90.2% (κ = 0.51-0.52) at the left side C4/5 level; 60.2% to 80.6% (κ = 0.50-0.54) at the right C5/6 level; 62.8% to 80.1% (κ = 0.52-0.55) at the left C5/6 level; 67.7% to 83.8% (κ = 0.50-0.64) at the right C6/7 level; and 62.2% to 79.4% (κ = 0.47-0.55) at the left C6/7 level, according to how the categories were grouped.
The average agreement between the five experienced readers and the agreement between the two inexperienced readers were also calculated, and the results were similar to those of the interobserver agreement among all readers (also shown in Table 2). The ICC for the experienced readers ranged from 0.69 to 0.75, indicating fair to excellent reproducibility, whereas the ICC for inexperienced readers was slightly lower than the average agreement among all seven readers (also shown in Table 2) and ranged from 0.61 to 0.66, indicating fair to moderate reproducibility.
The results of the analysis of intraobserver reliability based on percentage agreement and kappa statistics are also summarized in Table 3. For the distinction among the three individual grades at all six neural foramina, the ICC ranged from 0.83 to 0.95, indicating excellent agreement. The percentage agreement ranged from 85.4% to 97.9% (κ = 0.75-0.95) according to how the categories were grouped. The level of agreement was higher than the interobserver agreement.
According to our results, the MR grading system showed intraobserver and interobserver agreement at a sufficient level to serve as a reliable method for evaluating and reporting the degree of cervical NFS.
In a clinical setting, deciding the presence of cervical NFS or the severity of the stenosis may be more important than dividing the three different grades of stenosis. Our grading system could be further grouped to categorize between the presence and absence of stenosis and between non-severe and severe stenosis. In all of these groupings, the intraobserver and interobserver variability showed good reliability, suggesting the usefulness of the grading system in a clinical context with a diagram.
The interobserver variability was assessed between readers with different levels of experience who worked at different institutions. Considering the fact that a written description of the grading system with a diagram was provided to the readers and our study included diverse readers, the ICCs of 0.68 to 0.73 and the kappa values of 0.50 to 0.58 indicate fairly high levels of agreement. This means that the grading system is simple enough to be applied consistently and to be understood and learned. The average agreements among all seven readers and among the five experienced readers showed little difference, also suggesting that the system is easy to learn. The difference between the average agreement among all seven readers and the agreement between the two inexperienced readers could be explained by the good agreement between the experienced and inexperienced readers. It is difficult to compare the width of the extraforaminal nerve root with the width of the neural foramen because both the extraforaminal nerve root and the neural foramen are small structures. In addition, our study included MR images from outside hospitals. Interobserver agreement may be further enhanced by providing representative and educational cases of each grade. Intraobserver agreement in this study was assessed only with the readings of one observer. Based on the results that showed little difference between the average agreement among all seven readers and that among the five experienced readers, we assume that the intraobserver agreement would not be significantly affected by the reader's level of experience. The intraobserver agreement was high (the kappa values of 0.75 to 0.95), indicating that the grading system is easy enough to be applied consistently. The fair to good ICC values and moderate to substantial kappa values of interobserver and intraobserver agreement in our grading system suggest that the system is simple and reproducible.
The assessment of cervical NFS based on imaging studies has been attempted in various studies. However, most of these studies have focused on appropriate imaging techniques for detecting the presence of cervical NFS with MRI or computerized tomography (CT) imaging. Only a small number of studies have considered the assessment of stenosis severity. In a study by Bartlett et al. (9), a grading system (Grade 0, 1, or 2) of cervical NFS was suggested on imaging as follows: Grade 0, normal; Grade 1, osteophyte or disc touches the nerve root; and Grade 2 > 50% narrowing of foramen. For the grading system by Bartlett et al. (9), the definition of the width of the normal neural foramen was unclear, and thus 50% narrowing of the foramen was difficult to determine. In our grading system, we used the width of the extraforaminal nerve root in the same axial images as the reference standard, and thus the determination of 50% narrowing could be easily made. Ryan et al. (8) evaluated the severity of disease on T1-weighted and T2-weighted sequences. Neural impingements were graded as Grade 0, normal; Grade 1 ≥ 33% canal or neural foraminal encroachment; Grade 2 = 34-66% canal or neural foraminal encroachment; and Grade 3 > 66% canal or neural foraminal encroachment. The grading system by Ryan et al. (8) is heterogeneous and ambiguous as a result of including both the cervical canal and neural foraminal encroachment. A study by Song et al. (23) used NFS grading by MR and CT myelography. The grading criteria were as follows: Grade 0, normal; Grade 1, minimal; Grade 2, moderate (< 50% involvement of neural foramina); and Grade 3, severe (≥ 50% involvement of neural foramina). For that grading system, the definition of Grade 1 NFS was unclear. In addition, and as mentioned above, the definition of the width of the normal neural foramen was unclear, and thus 50% narrowing of the foramen was difficult to determine. Studies by Park et al. (1213) suggested NFS grading on the oblique sagittal T2-weighted sequence of MR imaging: Grade 0, normal; Grade 1, mild (< 50% involvement of the root circumference); Grade 2, moderate (> 50% involvement of root the circumference); and Grade 3, severe. This grading system showed high kappa values (0.71 to 0.99) and excellent reproducibility (12). However, for this system, an additional oblique sagittal T2-weighted sequence is needed. In addition, it is difficult to obtain a plane that is perfectly perpendicular to all cervical nerve roots. The strength of our new grading system is that we suggest a reference width for grading the severity of neural foraminal narrowing in the axial plane. In addition, a study by Park et al. (24) showed that the combined review of sagittal and axial views had the greatest diagnostic value (the kappa value for interobserver agreement was 0.606), and we suggest that the confidence and reliability of our grading system could be improved by adding the sagittal or oblique sagittal plane (24).
In the case of cervical NFS, the original width of the neural foramen and nerve root is difficult to define because the neural foramen is already narrowed and the nerve root is compressed in the foramen. Based on our experience, because cervical NFS was commonly induced by uncovertebral osteophytes that were medial to the extraforaminal nerve root and vertebral artery, the width of the extraforaminal nerve root at the level of the anterior margin of the superior articular process and the width between the vertebral artery and the superior articular process were usually not narrowed in most cases of NFS. We therefore used the width of the extraforaminal nerve root including the ipsilateral or contralateral side as references to define and grade NFS. On our assessment of the extraforaminal nerve root's visibility, only 13 neural foramina did not demonstrate the extraforaminal nerve root on axial T2-weighted images. Additionally, only one case did not demonstrate bilateral extraforaminal nerve roots at the C6/7 level, indicating that it is not difficult to find the extraforaminal nerve root on axial T2-weighted images. Some studies have reported anatomical variation in the vertebral artery that enters the transverse foramen of the cervical vertebrae in approximately 7% of cases (252627). An atypical level of entrance was usually observed with entrance into the C5 or C7. In our study, anatomical variation in the vertebral artery was not reported, and the width between the vertebral artery and superior articular process could be used alternatively as a reference. In cases of unclear bilateral extraforaminal nerve root and anatomical variation in the vertebral artery on axial MR images, we could consider the widest width of the cervical neural foramen or the length of the cervical pedicle as other references.
For an established classification system, the grading system information must not only be clear and simple but also have an influence on clinical practice. An earlier stage of stenosis with obliteration of less than 50% of the extraforaminal nerve root was defined as Grade 1, meaning that patients with Grade 1 stenosis are prone to progressing to higher grades of cervical NFS and radiculopathy because of the aging process. In these terms, it could be clinically important to detect these patients and provide appropriate medical attention.
Our study may be limited by a number of factors. Firstly, we did not evaluate the correlation between the stenosis grade and patient symptoms. The purpose of this study was to investigate the reliability of the new grading system for cervical NFS on axial T2-weighted MR images. Additional investigation is needed to elucidate the correlation between the grades and the severity of symptoms and therapeutic response. Secondly, the grading system was based only on axial T2-weighted images, and we focused on the simplicity of the system for its universal use. At some institutions in our country, axial T1-weighted images are not routinely scanned. Thirdly, this study was conducted at a single center, perhaps limiting its generalizability. To overcome this problem, we included MR images that had been imported from different hospitals. Finally, our study was based on recumbent MR images. Studies have shown that conventional recumbent MRI of the cervical spine may underestimate disease because the imaging is performed in a nondynamic, non-weight bearing position (282930). The upright MR system is currently not available in our institution, and, consequently, recumbent MR images were analyzed. However, we believe that the application of the new grading system does not necessarily have to be limited to recumbent MR images. The system may be applicable to upright MR images with sufficient interobserver and intraobserver reliability, although additional study is warranted.
In conclusion, the new MRI grading system provides a reliable assessment of cervical NFS.
Figures and Tables
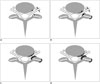 | Fig. 1Schematic diagrams of grading system for cervical neural foraminal stenosis in axial scans at intervertebral disc level in cervical spine.
A, B. Grade 0, normal-absence of neural foraminal stenosis with narrowest width of neural foramen (arrowheads) more than extraforaminal nerve root (black arrows). A shows no narrowing of neural foramen, and B shows mild narrowing. C. Grade 1, non-severe cervical neural foraminal stenosis, including narrowest width of neural foramen (arrowheads) same or less than (but more than 50% of) extraforaminal nerve root width. D. Grade 2, severe cervical neural foraminal stenosis, including narrowest width of neural foramen (arrowheads) same or less than 50% of extraforaminal nerve root width.
|
 | Fig. 264-year-old woman with Grade 0 cervical neural foraminal stenosis.Axial T2-weighted fast spin echo image shows bilateral C5/6 neural foramen without compromise. Arrowheads show width of right side extraforaminal nerve root. Arrows show narrowest width of right side neural foramen. Narrowest width of neural foramen is more than width of extraforaminal nerve root.
|
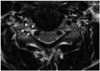
 | Fig. 371-year-old man with Grades 1 and 2 cervical neural foraminal stenosis, right and left sides, respectively.
A. Axial T2-weighted fast spin echo image shows compromised bilateral C6/7 neural foramina. In right side neural foramen, arrowheads show width of extraforaminal nerve root, and white arrows show right side of neural foramen width. Narrowest width of right side neural foramen (2.1 mm) is less than width of extraforaminal nerve root (2.5 mm) but more than 50% of width of extraforaminal nerve root. B. In left side neural foramen, arrowheads (2.5 mm) show width of contralateral extraforaminal nerve root, and white arrows show left side of neural foramen width (1.1 mm). Narrowest width of left side neural foramen is less than 50% of width of extraforaminal nerve root.
|
Table 1
Distribution of Cervical Neural Foraminal Stenoses

Table 2
Interobserver Reliability

Table 3
Intraobserver Reliability
References
1. Wainner RS, Gill H. Diagnosis and nonoperative management of cervical radiculopathy. J Orthop Sports Phys Ther. 2000; 30:728–744.
2. Yousem DM, Atlas SW, Goldberg HI, Grossman RI. Degenerative narrowing of the cervical spine neural foramina: evaluation with high-resolution 3DFT gradient-echo MR imaging. AJNR Am J Neuroradiol. 1991; 12:229–236.
3. Abbed KM, Coumans JV. Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery. 2007; 60(1 Supp1 1):S28–S34.
4. Modic MT, Masaryk TJ, Mulopulos GP, Bundschuh C, Han JS, Bohlman H. Cervical radiculopathy: prospective evaluation with surface coil MR imaging, CT with metrizamide, and metrizamide myelography. Radiology. 1986; 161:753–759.
5. Russell EJ. Cervical disk disease. Radiology. 1990; 177:313–325.
6. Jahnke RW, Hart BL. Cervical stenosis, spondylosis, and herniated disc disease. Radiol Clin North Am. 1991; 29:777–791.
7. Yousem DM, Atlas SW, Hackney DB. Cervical spine disk herniation: comparison of CT and 3DFT gradient echo MR scans. J Comput Assist Tomogr. 1992; 16:345–351.
8. Ryan AG, Morrissey BM, Newcombe RG, Halpin SF, Hourihan MD. Are T1 weighted images helpful in MRI of cervical radiculopathy? Br J Radiol. 2004; 77:189–196.
9. Bartlett RJ, Hill CR, Gardiner E. A comparison of T2 and gadolinium enhanced MRI with CT myelography in cervical radiculopathy. Br J Radiol. 1998; 71:11–19.
10. Modic MT, Masaryk TJ, Ross JS, Mulopulos GP, Bundschuh CV, Bohlman H. Cervical radiculopathy: value of oblique MR imaging. Radiology. 1987; 163:227–231.
11. Shim JH, Park CK, Lee JH, Choi JW, Lee DC, Kim DH, et al. A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J. 2009; 18:1109–1116.
12. Park HJ, Kim SS, Lee SY, Park NH, Chung EC, Rho MH, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol. 2013; 86:20120515.
13. Park HJ, Kim SS, Han CH, Lee SY, Chung EC, Kim MS, et al. The clinical correlation of a new practical MRI method for grading cervical neural foraminal stenosis based on oblique sagittal images. AJR Am J Roentgenol. 2014; 203:412–417.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990; 72:1178–1184.
15. Humphreys SC, Hodges SD, Patwardhan A, Eck JC, Covington LA, Sartori M. The natural history of the cervical foramen in symptomatic and asymptomatic individuals aged 20-60 years as measured by magnetic resonance imaging. A descriptive approach. Spine (Phila Pa 1976). 1998; 23:2180–2184.
16. Lee MJ, Riew KD. The prevalence cervical facet arthrosis: an osseous study in a cadveric population. Spine J. 2009; 9:711–714.
17. Tanaka N, Fujimoto Y, An HS, Ikuta Y, Yasuda M. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976). 2000; 25:286–291.
18. Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005; 85:257–268.
19. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003; 228:303–308.
20. Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol. 2011; 197:W134–W140.
21. Park HJ, Kim SS, Lee YJ, Lee SY, Park NH, Choi YJ, et al. Clinical correlation of a new practical MRI method for assessing central lumbar spinal stenosis. Br J Radiol. 2013; 86:20120180.
22. Rosner B. Fundamentals of Biostatistics. 7th ed. Canada: Cengage Learning;2010.
23. Song KJ, Choi BW, Kim GH, Kim JR. Clinical usefulness of CT-myelogram comparing with the MRI in degenerative cervical spinal disorders: is CTM still useful for primary diagnostic tool? J Spinal Disord Tech. 2009; 22:353–357.
24. Park MS, Moon SH, Lee HM, Kim TH, Oh JK, Lee SY, et al. Diagnostic value of oblique magnetic resonance images for evaluating cervical foraminal stenosis. Spine J. 2015; 15:607–611.
25. Shin HY, Park JK, Park SK, Jung GS, Choi YS. Variations in Entrance of Vertebral Artery in Korean Cervical Spine: MDCT-based Analysis. Korean J Pain. 2014; 27:266–270.
26. Bruneau M, Cornelius JF, Marneffe V, Triffaux M, George B. Anatomical variations of the V2 segment of the vertebral artery. Neurosurgery. 2006; 59:1 Suppl 1. ONS20–ONS24. discussion ONS20-ONS24
27. Rawal JD, Jadav HR. Anatomical study of variation of vertebral artery entering the foramen transversarium of cervical vertebrae. Natl J Med Res. 2012; 2:199–201.
28. Kitagawa T, Fujiwara A, Kobayashi N, Saiki K, Tamai K, Saotome K. Morphologic changes in the cervical neural foramen due to flexion and extension: in vivo imaging study. Spine (Phila Pa 1976). 2004; 29:2821–2825.
29. Vitaz TW, Shields CB, Raque GH, Hushek SG, Moser R, Hoerter N, et al. Dynamic weight-bearing cervical magnetic resonance imaging: technical review and preliminary results. South Med J. 2004; 97:456–461.
30. Jinkins JR, Dworkin JS, Damadian RV. Upright, weight-bearing, dynamic-kinetic MRI of the spine: initial results. Eur Radiol. 2005; 15:1815–1825.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download