Abstract
This pictorial review aims to illustrate the magnetic resonance imaging (MRI) findings and presentation patterns of anatomical variations and various benign and malignant pathologies of the duodenum, including sphincter contraction, major papilla variation, prominent papilla, diverticulum, annular pancreas, duplication cysts, choledochocele, duodenal wall thickening secondary to acute pancreatitis, postbulbar stenosis, celiac disease, fistula, choledochoduodenostomy, external compression, polyps, Peutz-Jeghers syndrome, ampullary carcinoma and adenocarcinoma. MRI is a useful imaging tool for demonstrating duodenal pathology and its anatomic relationships with adjacent organs, which is critical for establishing correct diagnosis and planning appropriate treatment, especially for surgery.
As a noninvasive imaging tool, magnetic resonance imaging (MRI) has the capabilites of multiplanar imaging and superior soft tissue contrast, which are very important in delineating the pathologies of the abdomen's solid and hollow organs. Duodenal lesions are generally detected incidentally, although various appearances are encountered radiologically. In accordance with the recent increased and widespread use of MRI in abdominal imaging, these often asymptomatic lesions are encountered more frequently today in patterns that some radiologists may not be familiar with.
In this pictorial review, patients who had undergone abdominal MRI for various indications were evaluated retrospectively, and those with duodenal lesions were selected. The MRI patterns of the duodenal lesions were assessed and analyzed.
Although it is normal that the most distal part of the common bile duct (CBD) and pancreatic duct cannot be visualized in magnetic resonance cholangiopancreatography (MRCP), sometimes it may be confused with real pathologies. These segments of the choledochus and the pancreatic duct are covered with muscle and belong to the Vaterian sphincter complex (1). A small intrasphincteric segment in these ducts or the existence of sphincter contractility may cause the Vaterian sphincter complex to not be visualized (12), which in turn causes false negative results in the diagnosis of small impacted calculus and papillary tumors. Moreover, severe contraction of the sphincter may cause what is called the "pseudo-calculus effect", which has a convex, semilunar shape in the distal CBD (Fig. 1) (1). There are phasic contractions approximately 4 times per minute that last 4.3 ± 0.5 seconds and that are revealed with manometric studies in the Vaterian sphincter complex, which normally has a basal pressure (12).
The major duodenal papilla is the slight mucosal bulging where the ampulla of Vater, created by the junction of the choledochus and the pancreatic duct, opens to the duodenum. The location, size and shape of the major papilla vary (3). The major papilla is in the 1/3 middle section of the descending segment of the duodenum at a rate of 75% and is located more distally in the horizontal segment at a rate of 25% (Fig. 2) (34).
The major duodenal papilla is the oval protrusion in the medial part of the descending segment of the duodenum. Although the papilla has a variable diameter, it is generally approximately 5-10 mm. The normal bulging of the papilla into the duodenum lumen is smaller than 1 cm (3). Normally, the papilla is scarcely differentiated from the duodenal mucosal folds around it (45). Although hypertrophic papilla can rarely occur as a normal variant, it may also develop in connection with inflammatory (acute cholangitis, acute pancreatitis, biliary calculus, periampullary diverticulum, infection-infestation, etc.) or neoplastic processes (intraductal papillary mucinous tumor, ampullary adenoma and tumor, periampullary cancer, etc.) (Fig. 3) (45).
A duodenal diverticulum is the herniation of the mucosa and the muscular layer from the intestine wall. A real duodenal diverticulum is congenital and arises from luminal recanalization anomalies during embryologic development (678910), and it includes all of the layers in the intestine wall. A pseudo-diverticulum, which is more frequently encountered, includes only the mucosa and submucosa layers and is most frequently found on the medial wall of the 2nd and 3rd segments of the duodenum (67810). Diverticula are most frequently seen in the duodenum, after the colon in the gastrointestinal (GI) tract (11). They are seen relatively more frequently in women, and the prevalence increases with age (9). Most cases are asymptomatic and are detected incidentally at a rate of approximately 11% during GI barium studies or during endoscopy that is performed for other reasons (911). Diverticulitis may be complicated with perforation in the retroperitoneal space (7). The area in which diverticula are most frequently seen is in the 2 to 3 cm periphery of the ampulla of Vater and are called juxtapapillary or periampullary diverticula. Diverticula in this location may rarely cause functional disorders in the ampulla and compression of the CBD, depending on their size and configuration, and in this way, may lead to biliopancreatic symptoms such as jaundice, cholangitis, or biliary calculus (911). If the diverticulum lumen is full of air or a mixture of air-liquid, it may be easily identified with computerized tomography (CT) or MRI. However, in the event that the lumen is completely filled with liquid, the diverticulum may be confused with cystic tumors of the pancreas or with choledochus cysts on CT or MRI (69). It is important to show the continuity in the duodenum lumen of a diverticulum with multiplanar imaging on MRI to avoid misinterpretation. Moreover, the air-liquid level is beneficial for identifying diverticula in axial sections and enabling their differentiation from the cystic lesions that may be seen in this location (Figs. 4, 5, 6) (69).
Intraluminal duodenal diverticulum (IDD) is one of the most rarely seen congenital anomalies of the duodenum, and it is thought to arise from defects in the recanalization process of the primitive foregut in the early gestational period (12131415). Typically, it is seen at the 2nd section of duodenum, near the papilla Vateri (1213). IDD is gender-free and is most frequently seen in the 3rd-5th decades (12). Although it is mostly asymptomatic, sometimes it may cause early satiety, bloating, nausea, and/or vomiting based on partial or total duodenal obstruction (16). Forty percent of cases may be accompanied with GI and extraintestinal malformations, such as annular pancreas, midgut malrotation, imperforate anus, choledochocele, or superior mesenteric artery (SMA) syndrome as well as extraintestinal anomalies such as congenital heart disease, bladder extrophy, or hypoplastic kidney (121314). In barium studies, IDD is seen as a pedunculated polypoid lesion that is projected into the real lumen and that is filled with barium, creating the appearance of what is called a pathognomonic "airport windsock" (Fig. 7) (101213). Lesions change place with peristaltism. On T2-weighted images, a hypointense rim that covers the diverticulum lumen inside the duodenum lumen (Fig. 7) is also a very important sign in diagnosis. This liquid collection, which is hypointense in its surrounding and which changes shape and place with intestinal peristaltism, is nearly pathognomonic for the IDD diagnosis on MRI and MRCP (12).
Annular pancreas is a rare congenital anomaly in which the pancreatic tissue band completely or partially surrounds the 2nd segment of the duodenum (17181920). The pancreas beings to develop from the 1 dorsal and 2 ventral buds as the outgrowth of the primitive foregut in the 4th-5th week of gestation. As the left ventral bud regresses, the right ventral bud rotates towards the dorsal as the duodenum expands, and it combines with the dorsal bud in the 7th week. As the ventral bud creates the head of the pancreas together with the uncinate process, the pancreatic body and tail are created from the dorsal bud (1819). Two theories are discussed in the development of annular pancreas. According to Lecco's theory, the left ventral bud regresses while the right ventral bud adheres on the duodenum wall and consequently becomes stretched and elongated together with a rotation towards the dorsal. According to Baldwin's theory, in contrast, the left ventral bud does not regress but rather moves in a contrary direction to that of the right ventral bud around the duodenum and they combine with the dorsal bud; in this way, the duodenum is covered with the pancreatic tissue (71819). Presentation of annular pancreas in children and in adults varies. Although vomiting caused by severe duodenal obstruction is the major symptom in the first year of life, 50% of adults are asymptomatic, and the condition is detected incidentally. The remaining 50%, however, show symptoms such as abdominal pain, vomiting, peptic ulcer, duodenal obstruction and pancreatitis in the 3rd-6th decades; however, the main presentation is pancreatitis (7181920). On MRI, the pancreatic tissue is recorded as completely or partially encircling the descending segment of the duodenum at the ampulla Vateri level or caudally (18). MRCP reveals an aberrant pancreatic duct encircling the duodenum (1819). This aberrant duct may be drained into the intrapancreatic part of the CBD or the Wirsung or Santorini ducts (Fig. 8) (20).
Duplication cysts arise from the recanalization defect in the duodenum lumen in the embryogenic period (7). They are very rare in the GI tractus, and approximately 12% occur in the stomach and duodenum (7). Duodenal duplications are well-circumscribed cystic masses that are seen more frequently in the 2nd and 3rd segments of the duodenum (Fig. 9) (721). These cysts are not typically communicated with the duodenum lumen (717). They are mostly incidental; however, sometimes they may also cause biliary obstruction and pancreatitis because of their location (717). Carcinoma may occur inside duplication cysts, although very rarely. For this reason, intracystic mural nodules and vegetation should be taken into consideration (722).
Choledochocele, which is the cystic dilatation of the intraduodenal segment of the choledochus, is concordant with type-III choledochal cysts according to Todani classification. It constitutes 4% of all choledochal cysts (Fig. 10) (23). Choledochocele is often encountered in elderly males. It generally presents with acute pancreatitis. Moreover, gallstones, cholangitis, cholecystitis are also seen. As choledochoceles protrude into the duodenum lumen, they may cause duodenal obstruction symptoms, hemorrhage and perforation. There exists a risk of pancreatic divisum and malignity, although the risk is low (232425).
Also known as Wilkie's syndrome, SMA syndrome is a rare pathology that arises from the compression of the 3rd segment of the duodenum intermittently between the aorta and the SMA, causing obstruction in the duodenum and proximal gastroduodenal dilatation (2627282930). It is more frequent in young adults and in women (28). Typical symptoms are epigastric pain, postprandial dyspepsia, nausea, vomiting of bile-stained material, early satiety and weight loss (262829). The most frequently encountered factor in etiopathogenesis is the decrease of superior mesenteric fatty tissue between the aorta and the SMA caused by extreme loss of weight. More rare reasons include short or abnormally inserted ligament of Treitz and hyperlordosis (282930). Normally, the fat pad between the aorta and the SMA increases the distance and angle between these two vascular structures and prevents the development of obstruction in the duodenum (30). It is stated in previous studies that the angle between the aorta and the SMA is 6-22° in those who have SMA syndrome and 25-60° in those who do not. Moreover, it is also found that the distance between the aorta and the SMA is shorter than normal in the duodenal crossing point in SMA syndrome (2-8 mm in SMA syndrome but 10-28 mm in normal cases) (Fig. 11) (2627).
Because the duodenum shows anatomic continuity with the pancreas, it is affected by the pathologies of the pancreas. Pancreatitis is the most frequently seen inflammatory process that affects the duodenum (710). Acute inflammation of the pancreas and excretion of exocrine enzymes may cause mild-severe duodenal edema, duodenal wall thickening and gastric outlet obstruction (Fig. 12) (710).
Peptic ulcer disease is the most common cause of benign duodenal stenosis. Duodenal ulcer may cause ectopic drainage of the CBD producing a hook-shaped configuration at its distal end (3132). In addition, benign conditions such as duodenal Crohn's disease and chronic pancreatitis, non-steroidal anti-inflammatory drugs, trauma, hyperplasia of Brunner's gland, corrosive agents, and malignant pathologies (duodenum, pancreas, gallbladder, ampulla) or metastases can cause duodenal stenosis or obstruction (Fig. 13) (31).
Indistinct fold (decrease in mucosal folds) is one of the imaging findings of duodenal involvement of celiac disease (6). Moreover, focal mucosal erosions, mural asymmetry, diffuse or nodular thickening in mucosal folds, mild luminal dilatation, stricture, and intramural fat storage are also reported (Fig. 14) (6).
Biliary tract fistulas are rare and may occur spontaneously or postoperatively. A long-term history of biliary stones, recurrent biliary tract infections, CBD stones, malignancy and previous biliary surgery are the most common causes of a choledochoduodenal fistula. In this situation, the biliary system is exposed to intestinal flora and fluid (33). It is reported that gallbladder cancer may develop secondary to the chemical irritation of this back flow via choledochoduodenal fistula (34). On plain films, air can be seen in the biliary tree (Fig. 15).
Choledochoduodenostomy is one of the biliary bypass procedures for treating benign biliary strictures or malignant obstruction of the biliary system. This operation is also indicated in patients with biliary fistulas, recurrent bile duct stones, stenosis of the sphincter of Oddi, and choledochal cysts (Fig. 16) (35).
The duodenum wall is a rare place of settlement for pancreatic pseudocysts. However, the inflammation and secretion that spread from the pancreas may cause the creation of pseudocysts on the posterior surface of the duodenum that are not covered by the peritoneum and that directly touch the head of pancreas. Because of this anatomic relationship, pseudocysts are most frequently seen in the 2nd segment (Fig. 17) (3637). A similar compression can be caused by rare diseases such as hydatid cysts (Fig. 18).
Peutz-Jeghers syndrome (PJS) is an autosomal dominant syndrome that is characterized by mucocutaneous pigmentation and hamartomatous GI polyps (38). Patients present with intestinal obstruction, abdominal pain, rectal hemorrhage, and/or intussusception. Full GI system involvement may occur except for the esophagus; however, PJS primarily affects the small intestines (738). Although the polyps may be very small, they may also reach a diameter of 3-4 cm, and they may be sessile or pedunculated. Polyps are histopathologically hamartoma (Fig. 19) (738).
Ampullary carcinomas originate from the glandular epithelium of the ampulla of Vater (5). Symptoms such as jaundice, abdominal pain, nausea, and vomiting occur relatively early, and for this reason, at the time of the diagnosis, ampullary carcinomas often manifest as small tumors (5). They are usually in polypoid or papillary form, and an infiltrative form can occur but is infrequent. On MRI, iso- or hypointense mass lesions that protrude into the duodenal lumen can be seen in T1- and T2-weighted images (Fig. 20) (5).
Adenocarciomas typically occur in the 5th and 6th decades, and they are the most common malignant tumors of the small intestine (6). More than 60% of small bowel malignant tumors arise in the duodenum (678). GI obstruction, bleeding and jaundice are the most common symptoms. Focal mural thickening, infiltrative annular strictures, and polypoid intramural or intraluminal masses are the imaging features on MRI (Fig. 21) (678).
Figures and Tables
 | Fig. 1Physiological sphincter contraction.On this MRCP image, major papilla (black arrow) appears very prominent at medial part of second section of duodenum (A). Distal choledochus is not visible (white arrow). Only after some relaxation of sphincter of Oddi did intramural segment of choledochus (arrow) become apparent (B). This image represents normal sphincter contractility. In order for morphologic changes and contractility in Vaterian sphincter complex to be evaluated optimally, they must be visualized both during contraction and during maximum relaxation. For this reason, it is necessary for patient to hold his or her breath in order to obtain serial MRCP images. MRCP = magnetic resonance cholangiopancreatography
|
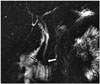 | Fig. 2Opening of distal end of choledochus to 3rd segment of duodenum.On this MRCP image, small periampullary diverticulum is depicted (black arrow), and also in this patient, major papilla variationally opens to 3rd section of duodenum (white arrow). Major papilla is in horizontal, i.e., 3rd, segment at rate of 25%. MRCP = magnetic resonance cholangiopancreatography
|
 | Fig. 3Prominent papilla.On coronal fast imaging employing steady-state acquisition MR image, nodular structure (arrow) is detected at level of major papilla (hypertrophic papilla). Normally, papilla is scarcely distinguished from surrounding duodenal mucosal folds, but as seen in this image, normal papillas may be seen as oval protruding structures of 5-10 mm. MRI and magnetic resonance cholangiopancreatography are important in hypertrophic papilla diagnosis and in detecting underlying pathology.
|
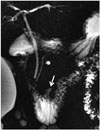 | Fig. 4Diverticulum.Diverticulum appears as fluid-filled pouch that protrudes from lumen of 3rd section of duodenum on this magnetic resonance cholangiopancreatography image (arrow). Diverticula are frequent in duodenum, and they are most often encountered in medial wall of 2nd and 3rd duodenal segments.
|
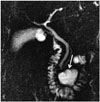 | Fig. 5Diverticulum.On MRCP, large diverticulum located distally in 3rd segment of duodenum is seen (arrow). If diverticula appear completely in fluid signal, they may misdiagnosed as cystic tumors of pancreas on CT or MRI. MRCP is highly successful both in imaging biliary tree and in determining liquid structures and their origins in this localization. MRCP = magnetic resonance cholangiopancreatography
|
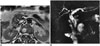 | Fig. 6Diverticulum.On axial T2-weighted (A) and magnetic resonance cholangiopancreatography (B) images, 4 cm diverticulum (white arrows in A) is seen at transverse segment of duodenum. On axial section (A), air within diverticulum lumen causes signal void (arrowheads in A). In addition, choledochus (arrow in B) is larger than expected, with filling defects within its lumen related to stones and stent (B). Air-fluid level within diverticulum is important sign that allows its recognition on axial sections and also allows differentiation from other cystic lesions that may be encountered within region. D = diverticulum
|
 | Fig. 7Intraluminal duodenal diverticulum (IDD) in patient with anal atresia corrected by surgery.In barium study, contrast-filled inpouching towards lumen (arrow) is detected at 2nd portion of duodenum with radiolucent rim (halo sign) (A). In same patient, magnetic resonance cholangiopancreatography (MRCP) image shows fluid collection surrounded by hypointense rim (arrow) in duodenal lumen (B). Intraluminal fluid collection on T2-weighted image with hypointense rim that changes shape with peristaltism on MRCP is almost characteristic sign for IDD. By means of multiplanar imaging, it is possible to view diverticulum wall in duodenum lumen and content of liquid with conventional MR images and MRCP without need for oral contrast agent.
|
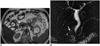 | Fig. 8Annular pancreas.On axial T2-weighted MR image (A), pancreatic tissue (black arrow) that covers postbulbar duodenum (white arrow) anterolaterally is detected in accordance with incomplete annular pancreas. On magnetic resonance cholangiopancreatography (MRCP) image of another patient (B), aberrant pancreatic duct is superimposed with 2nd portion of duodenum (arrow). Annular pancreas is abnormal pancreatic tissue band that covers 2nd part of duodenum circumferentially. If complete ring is formed, total duodenal obstruction after birth may occur. If ring is incomplete, obstruction may come to clinical attention much later or may be asymptomatic. MRCP is best non-invasive examination method that reveals ductal anatomy, and with MRI, pathologies can be detected such as chronic pancreatitis, pancreas divisum, and polysplenia that are highly encountered in annular pancreas compared with in general population.
|
 | Fig. 9Duplication cyst.On these coronal T2-weighted MR images, at lateral wall of 2nd portion of duodenum medial to gallbladder, smooth, thin-walled cystic structure (arrow) was noted. Duplication cysts are often encountered incidentally and may cause obstruction and pancreatitis because of their critical location. With MRI, cystic nature and relationship with adjacent structures can be revealed. Duplication cysts need to be differentiated from other cystic lesions (such as choledochal cysts and pancreatic pseudocysts) that may be seen in this location.
|
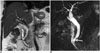 | Fig. 10Choledochocele.On coronal T2-weighted (A) and magnetic resonance cholangiopancreatography (B) images, cystic dilatation of distal end of choledochus is noted at level of major papilla (arrow). Cystic dilatation of intraduodenal segment of choledochus is called choledochocele, which is consistent with type-III choledochal cysts according to Todani classification.
|
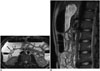 | Fig. 11Dilated duodenum secondary to superior mesenteric artery (SMA) syndrome.On axial (A) T2-weighted MR image, 2nd portion of duodenum is dilated and measures 4 cm (arrow). On sagittal image (B), it is detected that angle between aorta and SMA is narrowed (SMA syndrome). SMA syndrome can be diagnosed in clinically suspected cases on MRI by showing that aortomesenteric angle and distance are less than normal in reconstructed images and by dilatation proximal to obstruction. In barium studies, dilatation in duodenum, barium retention and vertical vascular external impression in 3rd segment are positive signs for SMA syndrome. However, these radiographic symptoms are non-specific, and they may also be seen in diseases such as scleroderma, diabetes, pancreatitis, and peptic ulcer.
|
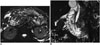 | Fig. 12Duodenal wall thickening secondary to acute pancreatitis.In patient with acute pancreatitis, on axial (A) and coronal (B) fat-suppressed T2-weighted MR images, mural thickening and edema in second portion of duodenum are depicted (white arrow in A, black arrow in B). Pancreatitis is most common inflammatory process that affects duodenum. Inflammation of pancreas and release of exocrine enzymes may lead to mild to severe duodenal edema and gastric outlet obstruction. Moreover, in severe pancreatitis, intramural hematoma may also develop.
|
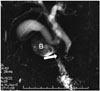 | Fig. 13Postbulbar stenosis.In this patient with gastrojejunostomy, postbulbar ulcer stenosis is shown on magnetic resonance cholangiopancreatography image. In addition, major papilla appears to be retracted cranially to bulbus, producing hook-shaped configuration. Intra- and extrahepatic biliary ducts appear mildly dilated. B = duodenal bulbus
|
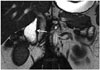 | Fig. 14Duodenal involvement of celiac disease.In this 32-year-old male patient with celiac disease, on coronal fast imaging employing steady-state acquisition MR image, mucosal folds of 2nd portion of duodenum appear indistinct and obscured (arrow). Among duodenal findings of celiac disease, decrease in number of mucosal folds, focal mucosal erosions, mural asymmetry, diffuse and nodular thickening of mucosal folds can be mentioned. Risk of adenocarcinoma and lymphoma increases in celiac patients, and MRI can be used for early diagnosis.
|
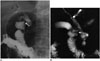 | Fig. 15Choledochoduodenal fistula.In this patient with jaundice, fistula tract between choledochus and duodenum (white arrow) is depicted on barium studies (A) and magnetic resonance cholangiopancreatography (MRCP) image (B). In barium study, filling of bile ducts with contrast medium through fistula is apparent (black arrows in A). Spontaneous bilioenteric fistulas most commonly occur secondary to gallstones and less often from peptic ulcer, malignancy and trauma. Barium studies are more informative and demonstrate fistula itself or reflux of contrast material into biliary system. MRI is valuable for depicting direct communication between biliary system and duodenum by means of MRCP, and it is also superior to other techniques in demonstrating primary pathology or underlying causes.
|
 | Fig. 16Choledochoduodenostomy.In this patient with chronic pancreatitis and repetitive cholangitis attacks, anastomosis between choledochus and duodenum (choledochoduodenostomy) is detected on magnetic resonance cholangiopancreatography (arrow). Intrahepatic bile ducts are mildly dilated and irregular because of cholangitis.
|
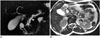 | Fig. 17Pseudocyst in duodenum wall.On magnetic resonance cholangiopancreatography (MRCP) (A) and axial T2-weighted MR images (B), cystic lesion consistent with pseudocyst is seen at lateral wall of duodenum in patient with chronic pancreatitis (black arrows in A and B). On MRCP image, pancreatic duct is dilated and irregular because of chronic pancreatitis (arrowhead in A). In pancreatitis, depending on depth of penetration, pseudocysts settle either between serosa and muscular layer or between muscular layer and mucosa. With accumulation of secretion and increase of pressure, obstruction in intestinal lumen and gastric outlet syndrome may occur. Tubular shape of pseudocyst that complies with progress of intestinal wall and abrupt flattening on intestine wall are findings that indicate intramural settlement. Duplication cysts and choledochocele should be considered in differential diagnosis.
|
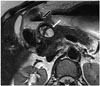 | Fig. 18External compression of duodenum by hydatid cyst.On T2-weighted axial MR image, multiloculated, thick-walled cystic lesion with internal septations is seen at level of pancreatic head-neck junction (white arrow). Lesion slightly compresses duodenum (black arrow) medially from right.
|
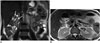 | Fig. 19Peutz-Jeghers syndrome (PJS).In this 41-year-old female patient, multiple polypoid lesions protruding into lumen (arrows) are seen in duodenum on coronal (A) and axial (B) T2-weighted MR images. On more caudal sections, many polyps are also present in jejunum, ileum and colon. PJS is also known as hamartomatous polyposis.
|
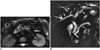 | Fig. 20Ampullary carcinoma.At level of ampulla of Vater, nodular mass that protrudes to lumen (arrow) is seen on fat-suppressed T2-weighted axial images (A). On magnetic resonance cholangiopancreatography (B), distal end of choledochus ends abruptly (white arrow) and bile ducts (arrowheads) and pancreatic duct (black arrow) are dilated because of papillary obstruction. If mass lesion cannot be discriminated on MRI, prominent duodenal papilla may be only sign of ampullary cancer. If marked or abrupt dilation of distal choledochus or pancreatic duct is encountered in patient who has no findings of gallstones or pancreatitis, ampullary carcinoma should be considered in differential diagnosis.
|
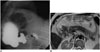 | Fig. 21Adenocarcinoma.Circumferential wall thickening of duodenum is detected on axial fast spin echo T2-weighted image (arrow in A) in 73-year-old patient with epigastric pain. In another patient, diffuse irregular mural thickening (arrow in B) causing luminal stenosis in 3rd and 4th segments of duodenum is demonstrated in MRI small-bowel follow-through image (histopathology: adenocarcinoma). In differential diagnosis of adenocarcinoma, carcinoid tumor, lymphoma, and metastasis should be taken into consideration.
|
References
1. Van Hoe L, Gryspeerdt S, Vanbeckevoort D, De Jaegere T, Van Steenbergen W, Dewandel P, et al. Normal Vaterian sphincter complex: evaluation of morphology and contractility with dynamic single-shot MR cholangiopancreatography. AJR Am J Roentgenol. 1998; 170:1497–1500.
2. Staritz M. Pharmacology of the sphincter of Oddi. Endoscopy. 1988; 20:Suppl 1. 171–174.
3. Horiguchi S, Kamisawa T. Major duodenal papilla and its normal anatomy. Dig Surg. 2010; 27:90–93.
4. Kim S, Lee NK, Lee JW, Kim CW, Lee SH, Kim GH, et al. CT evaluation of the bulging papilla with endoscopic correlation. Radiographics. 2007; 27:1023–1038.
5. Kim JH, Kim MJ, Chung JJ, Lee WJ, Yoo HS, Lee JT. Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics. 2002; 22:1335–1352.
6. Cronin CG, Lohan DG, DeLappe E, Roche C, Murphy JM. Duodenal abnormalities at MR small-bowel follow-through. AJR Am J Roentgenol. 2008; 191:1082–1092.
7. Jayaraman MV, Mayo-Smith WW, Movson JS, Dupuy DE, Wallach MT. CT of the duodenum: an overlooked segment gets its due. Radiographics. 2001; 21:S147–S160.
8. Zissin R, Osadchy A, Gayer G, Shapiro-Feinberg M. Pictorial review. CT of duodenal pathology. Br J Radiol. 2002; 75:78–84.
9. Mazziotti S, Costa C, Ascenti G, Gaeta M, Pandolfo A, Blandino A. MR cholangiopancreatography diagnosis of juxtapapillary duodenal diverticulum simulating a cystic lesion of the pancreas: usefulness of an oral negative contrast agent. AJR Am J Roentgenol. 2005; 185:432–435.
10. Hwang JI, Chiang JH, Yu C, Cheng HC, Chang CY, Mueller PR. Pictorial review: Radiological diagnosis of duodenal abnormalities. Clin Radiol. 1998; 53:323–332.
11. Tsitouridis I, Emmanouilidou M, Goutsaridou F, Kokozidis G, Kalambakas A, Papastergiou C, et al. MR cholangiography in the evaluation of patients with duodenal periampullary diverticulum. Eur J Radiol. 2003; 47:154–160.
12. Takamatsu S, Gabata T, Matsui O, Noto M, Ninomiya I, Nonomura A. Intraluminal duodenal diverticulum: MR findings. Abdom Imaging. 2006; 31:39–42.
13. Tu AS, Tran MH, Larsen CR. CT-appearance of intraluminal duodenal diverticulum. The "halo" sign. Comput Med Imaging Graph. 1998; 22:81–83.
14. Afridi SA, Fichtenbaum CJ, Taubin H. Review of duodenal diverticula. Am J Gastroenterol. 1991; 86:935–938.
15. Tasu JP, Rocher L, Amouyal P, Lorand I, Rondeau Y, Buffet C, et al. Intraluminal duodenal diverticulum: radiological and endoscopic ultrasonography findings of an unusual cause of acute pancreatitis. Eur Radiol. 1999; 9:1898–1900.
16. Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut. 1994; 35:557–559.
17. Berrocal T, Torres I, Gutiérrez J, Prieto C, del Hoyo ML, Lamas M. Congenital anomalies of the upper gastrointestinal tract. Radiographics. 1999; 19:855–872.
18. Lee NK, Kim S, Jeon TY, Kim HS, Kim DH, Seo HI, et al. Complications of congenital and developmental abnormalities of the gastrointestinal tract in adolescents and adults: evaluation with multimodality imaging. Radiographics. 2010; 30:1489–1507.
19. Sandrasegaran K, Patel A, Fogel EL, Zyromski NJ, Pitt HA. Annular pancreas in adults. AJR Am J Roentgenol. 2009; 193:455–460.
20. Türkvatan A, Erden A, Türkoğlu MA, Yener Ö. Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol. 2013; 14:905–913.
21. Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics. 1993; 13:1063–1080.
22. Rice CA, Anderson TM, Sepahdari S. Computed tomography and ultrasonography of carcinoma in duplication cysts. J Comput Assist Tomogr. 1986; 10:233–235.
23. Can MF, Kaymakc¸ioğlu N, Yağci G, Görgülü S, Tufan T. An adult choledochocele case presented with gastric outlet obstruction: a rare presentation. Turk J Gastroenterol. 2006; 17:70–73.
24. Jabłońska B. Biliary cysts: etiology, diagnosis and management. World J Gastroenterol. 2012; 18:4801–4810.
25. Lee HK, Park SJ, Yi BH, Lee AL, Moon JH, Chang YW. Imaging features of adult choledochal cysts: a pictorial review. Korean J Radiol. 2009; 10:71–80.
26. Konen E, Amitai M, Apter S, Garniek A, Gayer G, Nass S, et al. CT angiography of superior mesenteric artery syndrome. AJR Am J Roentgenol. 1998; 171:1279–1281.
27. Gustafsson L, Falk A, Lukes PJ, Gamklou R. Diagnosis and treatment of superior mesenteric artery syndrome. Br J Surg. 1984; 71:499–501.
28. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013; 105:236–238.
29. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012; 13:501–502.
30. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013; 15:189–191.
31. Al-Rashedy M, El-Dhuwaib Y, Issa M, Ballester P, Ammori BJ. Laparoscopic management of acquired benign duodenal strictures in adults. Internet J Surg. 2005; 6:14.
32. Lee HJ, Ha HK, Kim MH, Jeong YK, Kim PN, Lee MG, et al. ERCP and CT findings of ectopic drainage of the common bile duct into the duodenal bulb. AJR Am J Roentgenol. 1997; 169:517–520.
33. Dadzan E, Akhondi H. Choledochoduodenal fistula presenting with pneumobilia in a patient with gallbladder cancer: a case report. J Med Case Rep. 2012; 6:61.
34. Okabe T, Ohwada S, Ogawa T, Takeyoshi I, Sato Y, Kamoshita N, et al. Gallbladder carcinoma with choledochoduodenal fistula: a case report with surgical treatment. Hepatogastroenterology. 1999; 46:1660–1663.
35. Aramaki M, Ikeda M, Kawanaka H, Nishijima N, Tsutsumi N, Kano T, et al. Choledochoduodenostomy: simple side-to-side anastomosis. J Hepatobiliary Pancreat Surg. 2000; 7:486–488.
36. McCowin MJ, Federle MP. Computed tomography of pancreatic pseudocysts of the duodenum. AJR Am J Roentgenol. 1985; 145:1003–1007.
37. Bellon EM, George CR, Schreiber H, Marshall JB. Pancreatic pseudocysts of the duodenum. AJR Am J Roentgenol. 1979; 133:827–831.
38. Yano T, Yamamoto H. Vascular, polypoid, and other lesions of the small bowel. Best Pract Res Clin Gastroenterol. 2009; 23:61–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download