Abstract
Gadoxetate disodium is a widely used magnetic resonance (MR) contrast agent for liver MR imaging, and it provides both dynamic and hepatobiliary phase images. However, acquiring optimal arterial phase images at liver MR using gadoxetate disodium is more challenging than using conventional extracellular MR contrast agent because of the small volume administered, the gadolinium content of the agent, and the common occurrence of transient severe motion. In this article, we identify the challenges in obtaining high-quality arterial-phase images of gadoxetate disodium-enhanced liver MR imaging and present strategies for optimizing arterial-phase imaging based on the thorough review of recent research in this field.
Gadoxetate disodium (Eovist/Primovist; Bayer Healthcare, Berlin, Germany) is a recently introduced and valuable addition to magnetic resonance (MR) contrast agents for liver imaging. It enables vivid hepatobiliary phase imaging that reflects its specific hepatocyte uptake, it and can also provide dynamic phase imaging that is similar to the results from using conventional extracellular contrast agents (12). Although hepatobiliary phase images of gadoxetate disodium-enhanced MR offer new opportunities for detecting and characterizing focal hepatic lesions, arterial phase imaging remains critical for the diagnosis and treatment response evaluation of hepatic tumors such as hepatocellular carcinomas (HCCs) (345). Unfortunately, arterial-phase images of gadoxetate-enhanced liver MR are sometimes unsatisfactory because of weak arterial enhancement (67) and respiratory motion artifacts caused by so-called transient severe motion (TSM) (678910). In our article, we review the challenges in obtaining high-quality arterial-phase images and then explore possible solutions for optimizing the arterial-phase imaging of gadoxetate disodium-enhanced liver MR.
The smaller administered volume and gadolinium content of gadoxetate disodium compared with those of conventional extracellular MR contrast and the frequent occurrence of TSM can interfere with obtaining optimal arterial-phase images with gadoxetate disodium-enhanced MR (Table 1).
The standard gadoxetate-disodium injection dose is only half of that of conventional extracellular MR contrast (0.1 mL/kg of gadoxetate disodium vs. 0.2 mL/kg of gadopentetate dimeglumine) (11). The gadolinium content of a standard dose of gadoxetate-disodium is only a quarter of that of conventional extracellular contrast (0.025 mmol/kg of gadoxetate disodium vs. 0.1 mmol/kg of Gd-DTPA) (11). This recommended dose of gadoxetate disodum is based on a dose-finding study (12) that compared gadoxetate disodium given at three different gadolinium doses (0.0125, 0.025, and 0.05 mmol/kg) with gadopentetate dimeglumine at a standard gadolinium content of 0.1 mmol/kg. Compared with gadopentetate dimeglumine, similar enhancement characteristics of benign and malignant lesions during the first three minutes after contrast injection were found at 0.025 and 0.05 mmol/kg of gadoxetate disodium but not at the lowest dose of 0.0125 mmol/kg of (12).
In spite of the higher T1 relaxivity of gadoxetate disodium measured in human blood compared with other conventional extracellular MR contrasts (1314), this smaller administered volume and gadolinium content causes difficulties in obtaining consistently optimal arterialphase images. The smaller contrast volume can potentially shorten the enhancement duration of the contrast bolus and decrease the magnitude of the peak enhancement of the contrast bolus (Fig. 1) (1516). The lower gadolinium content can also result in a lower magnitude of the peak enhancement of the contrast bolus (1516). The effects of the small administered volume and gadolinium content are reflected in the weak arterial enhancement on images (67) and can pose challenges to capturing optimal arterial-phase imaging (10). Recent studies revealed that weak arterial enhancement can possibly affect the lesion conspicuity of hypervascular tumors such as HCCs and focal nodular hyperplasias during the arterial phase of gadoxetate-enhanced liver MR imaging in comparison with gadobenate dimeglumine-enhanced MR imaging (1718).
Recent work suggested that the image quality of the arterial phase in gadoxetate-enhanced liver MR is more commonly deteriorated by the TSM phenomenon than other gadolinium-based contrast agents (Fig. 2) (819). The reported incidence ranges from 10.7 to 18% (8192021), which suggests that over 10% of gadoxetate-enhanced liver MR examinations may have unsatisfactory arterial-phase images related to this artifact. As the name "TSM" implies, this artifact occurs in a very brief duration and is usually confined to the arterial phase (819202122).
The cause of this phenomenon remains unclear, although a number of recent articles have suggested possible risk factors related to the occurrence of TSM (192122). This artifact can potentially limit the advantages of gadoxetate-enhanced magnetic resonance imaging (MRI), although the impact of TSM on the diagnostic performance of gadoxetate-enhanced liver MRI has not been well demonstrated. Thus, strategies to minimize the effects of TSM need to be explored and are now an area of active research.
To compensate for the potential drawbacks of arterial-phase gadoxetate-enhanced liver MR imaging, i.e., weak arterial enhancement, difficulty capturing optimal arterial-phase images, and frequent occurrence of TSM, we can modify the injection protocol techniques to determine optimal scan timing and MR image acquisition methods (Table 1).
Given the half-sized injection volume and the quarter content of gadolinium compared with conventional extracellular MR contrast, the contrast bolus of gadoxetate-enhanced MR is likely to have a narrower shape and lower magnitude of peak enhancement (Fig. 1) (141516). It would be helpful to stretch out the duration of high-level aorta enhancement in the bolus shape to achieve proper arterial-phase timing and contrast-to-noise ratios of arterial hypervascular tumors such as HCC (Fig. 1). In order to obtain a favorable bolus shape, the following three approaches have been suggested in previous publications: 1) a decreased injection rate of 1 mL/sec; 2) an increased amount of gadoxetate; and 3) dilution of gadoxetate disodium by adding saline.
Prior reports (1114232425) compared the effect of a decreased injection rate of 1 mL/sec to those of a higher injection rate of 2 or 3 mL/sec commonly used for extracellular MR contrast. These studies (1114232425) demonstrated that the slower injection rate is beneficial not only in stretching the bolus but also in increasing the magnitude of the peak enhancement. This increased magnitude may look somewhat counterintuitive when we consider the contrast enhancement curve on CT (1516), in which an increased injection rate results in increased arterial enhancement. This discrepancy can be explained by the following two factors. First, because the bound fraction of gadoxetate disodium has a higher relaxivity than the unbound fraction (13), a slow injection rate likely permits sufficient time for gadoxetate disodium to be mixed with human blood and bound to plasma protein. Second, the relationship between MR signal intensity and gadolinium concentration is not linear, which might be responsible for saturation effects in a highly concentrated bolus related to T2* shortening whereas the relationship between CT attenuation and iodine concentration is linear. Nonetheless, excessive reduction of the injection rate may not have a positive effect on arterial-phase imaging: a previous study (26) that compared the injection rates of 0.5 mL/sec and 1 mL/sec showed a lower HCC contrast-to-noise ratio at an injection rate of 0.5 mL/sec than those of 1 mL/sec, although the result was not significant. Thus, given the results from previous work, a decreased injection rate of 1 mL/sec seems to the best rate for optimal arterial-phase imaging using gadoxetate disodium.
Although 0.025 mmol/kg of gadoxetate disodium is the on-label approved dose, the dose of gadoxetate disodium for optimal arterial-phase images is still under debate. Administration of gadoxetate disodium at a higher off-label dose such as a fixed dose of 10 mL (91027) or 20 mL (19) or a double dose (0.5 mmol/kg) (28) has been described in previous work. This higher dose would prolong the peak arterial perfusion time, which could help to overcome the temporal mismatch, increase the degree of the peak aortic enhancement, and consequently improve liver-to-lesion contrast on arterial-phase images (1428). However, the safety of this higher off-label dose has not been demonstrated. A recent study reported that when a fixed 20-mL dose of gadoxetate disodium was used, TSM occurred more frequently than when the standard dose was used (19). Thus, even though administering gadoxetic acid at a fixed dose of 10 mL seems to be preferred in many institutions (91027), it should be noted that it is an off-label dose.
Diluting gadoxetate disodium with saline has been proposed as a possible option to help stretch the contrast bolus (29) because can possibly have similar effects to those of a decreased injection rate on a bolus shape. However, previous research (11) has demonstrated limited evidence of benefits. Given a risk of contamination though the mixing process, dilution is not well regarded as a practical solution for improving the quality of arterial-phase imaging.
Gadoxetate disodium-enhanced liver MR demands more precise timing for hepatic arterial-phase images because of the narrower contrast bolus compared with conventional extracellular MR contrast. Because a simple fixed delay method is prone to timing error with the influence of individual variation or injection protocol, it is not recommended in gadoxetate disodium-enhanced liver MRI. Previous work showed that simple fixed delay was inferior to bolus tracking (2330) or test bolus (29) in capturing proper hepatic arterial-phase timing, aortic enhancement, and lesion-to-liver contrast in hypervascular HCCs. One study suggested that the optimal scan delay for hypervascular HCCs in gadoxetate-enhanced arterial-phase images is 7-12 seconds after the peak aortic enhancement at an injection rate of 1 mL/sec (31). One possible drawback of manual bolus tracking is its operator-dependent nature. If the procedure is conducted by well-trained technicians, the best results can be achieved; optimal arterial-phase images were obtained in 91.7% of patients using manual bolus-tracking (23). The recent introduction of an automated bolus detection method appears to be promising for solving the potential problems with manual bolus tracking (32). Applying a test bolus method may be limited by the small amount of gadoxetate disodium and rapid uptake by hepatocytes, which can possibly leave an insufficient amount of the contrast and thus deteriorate lesion-to-liver contrast (10). Another option can be acquiring multiple (usually three) arterial-phase images with a fixed time delay (20). In addition to a high rate of adequate arterial-phase images (98%) (20), this method can provide robustness to TSM and the availability of more than one arterial-phase value, which can possibly provide a more precise assessment of the arterial enhancement of focal hepatic lesions. However, because of the higher temporal resolution required for multiple arterial-phase images, reducing the spatial resolution and the signal-to-noise ratio results in inevitable trade-offs (20).
Therefore, simple fixed delay is discouraged for determining optimal arterial-phase scan timing for gadoxetate-enhanced liver MR. Bolus tracking or acquiring multiple (usually three) arterial-phase images with a fixed time delay can be better strategies.
Currently, a fat-suppressed three-dimensional (3D), T1-weighted gradient echo (GRE) sequence is widely accepted as a key sequence for liver dynamic MR because it provides satisfactory spatial resolution, adequate signal-to-noise ratio and a temporal resolution that is sufficient to be performed during breath holding (223334). Volumetric interpolated breath hold examination (VIBE; Siemens Healthcare, Erlangen, Germany), liver acquisition volume acceleration (LAVA; GE Healthcare, Milwaukee, WI, USA), and enhanced T1-high resolution isotropic volume excitation (eTHRIVE; Philips Healthcare, Best, the Netherlands) are commonly used 3D GRE sequences for liver MR examinations (35). Most institutions employ parallel acquisition techniques decrease acquisition time or improve spatial resolution (3637). The use of multiple arterial-phase acquisitions (20), controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) VIBE and radial k-space sampling 3D GRE sequence (as known as Radial-VIBE) (30383940) can be considered techniques to improve the quality of arterial-phase images.
Clinical use of 3T MR imaging has evolved rapidly over the past decade. The major advantage of 3T is a nearly two-fold signal gain in signal-to-noise ratio, which allows higher signal, higher spatial resolution or shorter examination times in liver imaging compared with 1.5T (4142). In addition, theoretically substantial gains can be anticipated in post-contrast T1-weighted imaging at 3T. T1 relaxation times are prolonged as the magnetic field increases; however, the T1 relaxation times of gadolinium contrast increase minimally whereas the values of hepatic tissue can lengthen substantially (4142). Thus, it likely leads to an apparent contrast gain on high-field MR imaging, which brings higher lesion-to-liver contrast in hypervascular tumors such as HCC in arterial-phase imaging (Fig. 3). However, there is a paucity of data that compare the arterial-phase image quality of gadoxetate disodium-enhanced liver MRI in different field strengths. Although higher field imaging has some disadvantages including specific absorption rate limitation, increased susceptibility artifacts, field inhomogeneity and standing wave artifacts, we believe that 3T MR imaging is a promising approach to improving the arterial-phase image quality of gadoxetate disodium-enhanced liver MRI. Given improved signal-to-noise ratio and better lesion-to-liver contrast, additional studies are warranted to prove the potential advantages of 3T on arterial-phase images of gadoxetate-disodium enhanced liver MRI for detection and characterization, especially in small lesions.
In order to develop strategies to avoid TSM, it is critical to identify TSM causes and risk factors. Unfortunately, TSM's causes and pathophysiology have yet to be elucidated. Recent studies suggested a number of possible risk factors for TSM: a previous episode of TSM (2122), an off-label administered volume of 20-mL fixed dose at 2 mL/s (19), a history of allergy to iodinated contrast for CT (21), low body weight (19), and chronic obstructive lung disease (COPD) (19). However, some of the risk factors are still in question because the results from the published studies were not consistent; low body weight (1921) and the presence of COPD (2122) were not identified as significant risk factors in some studies.
Some studies described ringing artifacts as a different type of artifact that can also occur during the arterial phase of gadoxetate disodium-enhanced liver MRI because of rapid changes in the contrast concentration while obtaining the data in the center of the k-space (43). TSM ringing artifacts and motion artifacts may have similar appearances on images, and some earlier studies did not seem to specifically describe these artifacts. However, by definition, ringing artifacts are centered on the vessels and confined to the abdomen, whereas TSM motion artifacts are centered on the abdominal wall and can extend outside of it (2243). In our experience, TSM motion artifacts seem to be more commonly encountered and more problematic than ringing artifacts in clinical practice.
In patients with high risk factors for TSM, it could be better to consider using other contrast agent in cases in which optimal arterial-phase images as well as hemodynamic information are crucial for characterizing lesions, for example, when we evaluate a lesion that is suspicious for hepatic hemangioma. However, in cases in which hepatobiliary phase images are essential, such as detecting hepatic metastasis in patients with underlying malignancy, we can choose gadoxetate disodium as the MR contrast at the same time that we attempt to minimize the effects of TSM on arterial-phase images. The use of multiple arterial-phase acquisition can overcome the effect of TSM because at least one arterial-phase image can be obtained well-timed and not compromised by motion in 81% of patients with TSM (20). The use of motion-insensitive sequences such as CAIPIRINHA VIBE and radial-VIBE can be promising (30383940).
Owing to technical advances in image registration techniques, subtraction images have increasingly become an integral part of liver MRI in many hospitals (4445). As was demonstrated with other gadolinium contrast agents (4445), subtraction images between the unenhanced and arterial phase using gadoxetate disodium can be more beneficial for depicting the arterial enhancement of lesions than visual image comparison, which resulted in higher diagnostic performance in small HCCs less than 3 cm (46). Subtraction images would be helpful for overcoming the potential weak arterial enhancement of gadoxetate disodium-enhanced liver MRI. However, one of the limitations in subtraction images is the susceptibility to misregistration artifacts because the respiratory motion causes erroneous co-registration between source images in the subtraction process (46). We should keep in mind that misregistration artifacts can yield pseudoenhancement of lesions and thus overdiagnosis of HCC (44).
Gadoxetate disodium-enhanced MR can greatly improve the detection and characterization of focal liver lesions because of its distinctive properties as a dual-function contrast agent. However, these advantages are sometimes diminished by the poor quality of arterial-phase imaging owing to weak arterial enhancement, inappropriate scan timing, and severe motion artifacts. The main causes of these difficulties were explained by the small injection volume and gadolinium content as well as the occurrence of TSM in more than 10% of patients. In this article, we reviewed possible strategies for overcoming these problems. Many institutions employ a modified injection protocol with a slow injection rate of 1 mL/sec and a fixed increased dose of 10 mL to obtain a favorable contrast bolus. To determine the optimal arterial-phase timing, bolus detection or multiple arterial-phase acquisition with a fixed delayed method is a preferred option. In patients at high risk for developing TSM, i.e., a previous episode of TSM (2122), a higher off-label administered volume of 20-mL fixed dose at 2 mL/s (19), a history of allergy to iodinated contrast for CT (21), low body weight (19), or COPD (19), we should determine whether a conventional MR contrast agent may be preferred over gadoxetate disodium by weighing the benefits of the hepatobiliary phase, i.e., high lesion-to-liver contrast against the risk of TSM. The use of multiple arterial-phase acquisition or new motion-resistant sequences would help to mitigate the effects of TSM on arterial-phase images. For a better depiction of arterial hypervascularity of lesions, either 3T or subtraction techniques are favored.
Figures and Tables
Fig. 1
Simulated contrast enhancement curve of abdominal aorta and liver.
Small amount of administered volume and gadolinium content of gadoxetate disodium (red line) can potentially shorten enhancement duration of contrast bolus and decrease magnitude of peak enhancement of contrast bolus compared with favorable contrast bolus curve (green line). This shape of gadoxetate disodium curve (red line) can pose challenges in capturing optimal arterial phase imaging.

Fig. 2
68-year-old man with respiratory motion artifacts.
This arterial phase image of gadoxetate-enhanced liver MR (B) is severely contaminated by respiratory motion artifacts related to TSM, whereas noncontrast (A), portal venous (C), and hepatobiliary (D) phase images look fine. Owing to presence of TSM, arterial hypervascularity of lesion in right lobe of liver (arrows) cannot be exactly evaluated on this MR image, although lesion (arrows) is clearly defined on non-contrast (A), portal venous (C), and hepatobiliary (D) phase images. Lesion was confirmed as HCC after surgery. HCC = hepatocellular carcinoma, TSM = transient severe motion
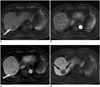
Fig. 3
71-year-old man who underwent liver MRI both at 1.5T and 3T in one-week interval.
On arterial phase images at 3T (A, B), four more lesions (arrows) as well as large hypervascular tumor (arrowheads) are additionally detected, whereas on 1.5T MR images (C, D), only main lesion (arrowheads) is found. Lesions look more conspicuous on 3T MR images (A, B) than they do 1.5T MR images (C, D). Non-enhanced follow-up CT images (E, F) after transarterial chemoembolization demonstrate lipidol uptakes on four small lesions (arrows) and main lesion (arrowheads).
Table 1
Challenges and Solutions for Optimal Arterial Phase Images with Gadoxetate-Enhanced Liver MRI

References
1. Kudo M. Will Gd-EOB-MRI change the diagnostic algorithm in hepatocellular carcinoma? Oncology. 2010; 78:Suppl 1. 87–93.
2. Fowler KJ, Brown JJ, Narra VR. Magnetic resonance imaging of focal liver lesions: approach to imaging diagnosis. Hepatology. 2011; 54:2227–2237.
3. Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015; 61:1056–1065.
4. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
5. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.
6. Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol. 2009; 50:709–715.
7. Tamada T, Ito K, Sone T, Yamamoto A, Yoshida K, Kakuba K, et al. Dynamic contrast-enhanced magnetic resonance imaging of abdominal solid organ and major vessel: comparison of enhancement effect between Gd-EOB-DTPA and Gd-DTPA. J Magn Reson Imaging. 2009; 29:636–640.
8. Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013; 266:452–461.
9. Cruite I, Schroeder M, Merkle EM, Sirlin CB. Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol. 2010; 195:29–41.
10. Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol. 2010; 195:13–28.
11. Tamada T, Ito K, Yoshida K, Kanki A, Higaki A, Tanimoto D, et al. Comparison of three different injection methods for arterial phase of Gd-EOB-DTPA enhanced MR imaging of the liver. Eur J Radiol. 2011; 80:e284–e288.
12. Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996; 200:59–67.
13. Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005; 40:715–724.
14. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009; 44:305–310.
15. Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010; 256:32–61.
16. Bae KT. Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology. 2003; 227:809–816.
17. Tirkes T, Mehta P, Aisen AM, Lall C, Akisik F. Comparison of Dynamic Phase Enhancement of Hepatocellular Carcinoma Using Gadoxetate Disodium vs Gadobenate Dimeglumine. J Comput Assist Tomogr. 2015; 39:479–482.
18. Kim HJ, Kim BS, Kim MJ, Kim SH, de Campos RO, Hernandes M, et al. Enhancement of the liver and pancreas in the hepatic arterial dominant phase: comparison of hepatocyte-specific MRI contrast agents, gadoxetic acid and gadobenate dimeglumine, on 3 and 1.5 Tesla MRI in the same patient. J Magn Reson Imaging. 2013; 37:903–908.
19. Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014; 272:123–131.
20. Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014; 271:426–434.
21. Kim SY, Park SH, Wu EH, Wang ZJ, Hope TA, Chang WC, et al. Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol. 2015; 204:1220–1227.
22. Bashir MR, Castelli P, Davenport MS, Larson D, Marin D, Hussain HK, et al. Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology. 2015; 274:141–148.
23. Haradome H, Grazioli L, Tsunoo M, Tinti R, Frittoli B, Gambarini S, et al. Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging. 2010; 32:334–340.
24. Chung SH, Kim MJ, Choi JY, Hong HS. Comparison of two different injection rates of gadoxetic acid for arterial phase MRI of the liver. J Magn Reson Imaging. 2010; 31:365–372.
25. Schmid-Tannwald C, Herrmann K, Oto A, Panteleon A, Reiser M, Zech C. Optimization of the dynamic, Gd-EOB-DTPA-enhanced MRI of the liver: the effect of the injection rate. Acta Radiol. 2012; 53:961–965.
26. Kim SM, Heo SH, Kim JW, Lim HS, Shin SS, Jeong YY, et al. Hepatic arterial phase on gadoxetic acid-enhanced liver MR imaging: a randomized comparison of 0.5 mL/s and 1 mL/s injection rates. Korean J Radiol. 2014; 15:605–612.
27. Bashir MR, Gupta RT, Davenport MS, Allen BC, Jaffe TA, Ho LM, et al. Hepatocellular carcinoma in a North American population: does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? J Magn Reson Imaging. 2013; 37:398–406.
28. Motosugi U, Ichikawa T, Sano K, Sou H, Onohara K, Muhi A, et al. Double-dose gadoxetic Acid-enhanced magnetic resonance imaging in patients with chronic liver disease. Invest Radiol. 2011; 46:141–145.
29. Motosugi U, Ichikawa T, Sou H, Sano K, Ichikawa S, Tominaga L, et al. Dilution method of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI). J Magn Reson Imaging. 2009; 30:849–854.
30. Park YS, Lee CH, Kim IS, Kiefer B, Woo ST, Kim KA, et al. Usefulness of controlled aliasing in parallel imaging results in higher acceleration in gadoxetic acid-enhanced liver magnetic resonance imaging to clarify the hepatic arterial phase. Invest Radiol. 2014; 49:183–188.
31. Goshima S, Kanematsu M, Kondo H, Watanabe H, Kawada H, Moriyama N, et al. Evaluation of optimal scan delay for gadoxetate disodium-enhanced hepatic arterial phase MRI using MR fluoroscopic triggering and slow injection technique. AJR Am J Roentgenol. 2013; 201:578–582.
32. Hussain HK, Londy FJ, Francis IR, Nghiem HV, Weadock WJ, Gebremariam A, et al. Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradient-recalled-echo sequence: comparison with test-bolus method. Radiology. 2003; 226:558–566.
33. Lee VS, Lavelle MT, Rofsky NM, Laub G, Thomasson DM, Krinsky GA, et al. Hepatic MR imaging with a dynamic contrast-enhanced isotropic volumetric interpolated breathhold examination: feasibility, reproducibility, and technical quality. Radiology. 2000; 215:365–372.
34. Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999; 212:876–884.
35. Guglielmo FF, Mitchell DG, Gupta S. Gadolinium contrast agent selection and optimal use for body MR imaging. Radiol Clin North Am. 2014; 52:637–656.
36. McKenzie CA, Lim D, Ransil BJ, Morrin M, Pedrosa I, Yeh EN, et al. Shortening MR image acquisition time for volumetric interpolated breath-hold examination with a recently developed parallel imaging reconstruction technique: clinical feasibility. Radiology. 2004; 230:589–594.
37. Vogt FM, Antoch G, Hunold P, Maderwald S, Ladd ME, Debatin JF, et al. Parallel acquisition techniques for accelerated volumetric interpolated breath-hold examination magnetic resonance imaging of the upper abdomen: assessment of image quality and lesion conspicuity. J Magn Reson Imaging. 2005; 21:376–382.
38. Kim KW, Lee JM, Jeon YS, Kang SE, Baek JH, Han JK, et al. Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with K-space weighted image contrast (KWIC). Eur Radiol. 2013; 23:1352–1360.
39. Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, et al. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014; 24:1290–1299.
40. Budjan J, Ong M, Riffel P, Morelli JN, Michaely HJ, Schoenberg SO, et al. CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE) for dynamic liver imaging: comparison of gadoterate meglumine, gadobutrol and gadoxetic acid. Eur J Radiol. 2014; 83:2007–2012.
41. Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am. 2007; 15:277–290. v
42. Boll DT, Merkle EM. Imaging at higher magnetic fields: 3 T versus 1.5 T. Magn Reson Imaging Clin N Am. 2010; 18:549–564. xi–xii.
43. Tanimoto A, Higuchi N, Ueno A. Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci. 2012; 11:91–97.
44. Seçil M, Obuz F, Altay C, Gencel O, Igğci E, Sagğol O, et al. The role of dynamic subtraction MRI in detection of hepatocellular carcinoma. Diagn Interv Radiol. 2008; 14:200–204.
45. Yu JS, Kim YH, Rofsky NM. Dynamic subtraction magnetic resonance imaging of cirrhotic liver: assessment of high signal intensity lesions on nonenhanced T1-weighted images. J Comput Assist Tomogr. 2005; 29:51–58.
46. An C, Park MS, Kim D, Kim YE, Chung WS, Rhee H, et al. Added value of subtraction imaging in detecting arterial enhancement in small (<3 cm) hepatic nodules on dynamic contrast-enhanced MRI in patients at high risk of hepatocellular carcinoma. Eur Radiol. 2013; 23:924–930.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download