Abstract
We report a rare case of primary pulmonary low-grade angiosarcoma on dynamic contrast-enhanced CT and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT imaging. A 38-year-old, asymptomatic woman was hospitalized because of an abnormality on chest radiography. A dynamic contrast-enhanced chest CT showed a 1.2 cm-sized irregular-margined nodule with strong and persistent enhancement in the right lower lobe. The lesion had low metabolic activity on an 18F-FDG PET/CT scan. The patient underwent a wedge resection for the lesion, and pathology revealed a primary pulmonary low-grade angiosarcoma.
Angiosarcoma is a rare malignant tumor that is derived from vascular endothelium and accounts for about 1-2% of all soft tissue tumors. Soft tissue and skin are its common primary sites. The most frequent visceral organs that are affected include the heart (generally, the right atrium), liver, and spleen (1234). Pulmonary angiosarcomas are almost always metastatic malignancies from other primary sites. Primary pulmonary angiosarcomas are characterized by insidious growth and extensive local invasion by the time of diagnosis (14).
Because primary pulmonary angiosarcomas are rare, little is known about the imaging features of primary pulmonary angiosarcoma on dynamic contrast-enhanced computed tomography (CT) and with 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT imaging. We report a rare case of primary low-grade angiosarcoma on dynamic contrast-enhanced CT and 18F-FDG PET/CT imaging. This report was approved by the Institutional Review Board and written informed consent was waived.
A 38-year-old woman visited our institution because of an abnormal chest radiograph on a routine health checkup at another hospital. The patient did not have any respiratory symptoms and did not have history of smoking. All laboratory findings were within the normal limits.
Dynamic contrast-enhanced CT and 18F-FDG PET/CT imaging were performed. For the dynamic CT scan, before the intravenous injection of contrast medium, a phase of 13 images was obtained throughout the nodule for 30 mm along the z-axis, with 2.5-mm collimation, 120 kVp, 90 mAs, 0.8-second gantry rotation time, and a table speed of 3.75 mm/sec over 8 seconds. An additional three phases of images were obtained at 60, 120, and 180 seconds after an iomeprol injection (Iomeron 300; Bracco, Milan, Italy), which was modified from a previous study that described a dynamic CT evaluation of solitary pulmonary nodules (5). The contrast injection was administered at a rate of 3 mL/sec for a total of 1.8 mL/kg using a power injector. Image data were reconstructed with a thickness of 2.5 mm using a standard algorithm. Helical CT scans (5-mm collimation, 120 kVp, 125 mAs, 0.8-second gantry rotation time, table speed of 15 mm/sec) were obtained from the lung apices to the level of the middle pole of both kidneys immediately after dynamic imaging at 140 seconds.
Contrast-enhanced chest CT scans (Fig. 1A-E) indicated a 1.2 cm-sized nodule in the right lower lobe, which showed needle-like projections in the periphery of the nodule. The nodule showed early strong enhancement with a peak enhancement of 245 Hounsfield units (HU) and a gradual loss of enhancement (washout) to 168 HU through 180 seconds (Fig. 1F). 18F-FDG PET/CT (Fig. 1G) indicated a nodule with mildly increased radiopharmaceutical uptake that reached a maximum standardized uptake value (SUVmax) of 2.0.
A video-assisted thoracoscopic surgery was performed on the nodule, and a low-grade angiosarcoma was diagnosed with histopathology (Fig. 1H-M). The gross appearance of the nodule was soft, dark red tissue with hemorrhage. Microscopically, the tumor was highly vascular, and higher power imaging showed prominent, freely-anastomosing vascular channels, papillary growth, and endothelial tufting that was absent to minimal. The CT scan revealed needle-like projections in the periphery of the nodule that corresponded with vasoformative features that were anastomosing vascular channels lined by malignant endothelium. Immunohistochemistry staining revealed that the tumor cells were diffusely positive for smooth muscle actin (SMA) and CD31, was well as weakly positive for Ki-67. After surgery, adjuvant chemotherapy was planned and the patient was discharged. The patient recovered well post-operatively and no clinical or radiological evidence of recurrence were noted at the two-year follow-up.
Primary vascular neoplasms of the lung are rare. Most of these lesions have only been reported as isolated case studies or as a small series. Currently, there are about 20 reports on primary pulmonary angiosarcomas in the English literature (14). We conducted an English literature review and found four cases of pulmonary angiosarcomas in which CT images of the disease were described (summarized in Table 1). Common radiographic presentation may indicate that there are multiple peripheral pulmonary nodules. However, there were no characteristics that would differentiate these nodules from other types of metastatic diseases.
In this case, the tumor had a pure solitary pulmonary nodule, which was an unusual finding (236). In addition, mismatch between imaging features of the dynamic contrast-enhanced CT scan and PET/CT scan actually reflected the pathologic features of this tumor. Contrary to the general concept that FDG accumulation may be correlated with angiogenesis along with higher CT enhancement, in the present study, the tumor had low metabolic activity but showed high angiogenesis on dynamic CT. The dynamic CT images indicated that the mean attenuation values of the nodule was 249 HU at 30 seconds after the contrast injection and 194 HU 60 seconds after the injection. In contrast, the SUVmax was 2.0 on 18F-FDG PET. Therefore, the characteristics on dynamic contrast-enhanced CT scans are likely correlated with histological features, including blood-filled vascular spaces and vascular channels. The pattern of enhancement, early strong enhancement, and delayed washout, indicates that the tumor has a vascular origin, and its mild FDG uptake suggests it to be a highly vascular and low-grade tumor. Moreover, on pathology, multiple needle-like projections in the periphery of the nodule correlated with the innumerable anastomosing vascular channels lined by malignant endothelium showing vasoformative features.
Meta-analysis results of a previous study indicated that FDG-PET can discriminate between sarcomas and benign tumors and low- and high-grade sarcomas based on the mean SUV (789). Cucci et al. (10) described a case of a patient with low-grade breast angiosarcoma. The patient's follow-up CT showed a retroperitoneal right pelvic lymph node with low metabolic activity on FDG-PET (SUVmax, 1.15). The prognosis depends on the histologic grade of the tumor. Patients with low-grade tumors tend to have higher survival rates and lower recurrence rates (11).
To our knowledge this is the first report of a primary pulmonary low-grade angiosarcoma that was diagnosed with a dynamic chest CT and 18F-FDG PET/CT. The morphology, pattern of dynamic enhancement, and SUVmax of the lesion helped diagnose the lesion and correlated with histopathological features and the aggressiveness of the tumor. Therefore, dynamic chest CT and 18F-FDG PET/CT can be useful for evaluating low-grade vascular neoplasms in lungs.
Figures and Tables
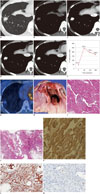 | Fig. 138-year-old woman with primary pulmonary angiosarcoma.
A. Transverse lung-window (window level of -700 H and window width of 1500 H) CT image indicates 1.2 cm-sized nodule in right lower lobe with spiculated margin and needle-like projections (arrows) in periphery of nodule. B-F. Serial transverse images obtained through nodule for 180 seconds allowed dynamic enhancement curve for nodule to be plotted. Graph shows early peak enhancement and gradual loss of enhancement (washout). G. 18F-fluorodeoxyglucose positron emission tomography fused axial image shows nodule with mildly increased radiopharmaceutical uptake (arrow) with maximum standardized uptake value of 2.0. H. Gross photograph indicates soft and dark-reddish nodule with hemorrhage (arrows). I. Photomicrograph reveals highly vascular tumor with prominent freely anastomosing vascular channels, papillary growth, and endothelial tufting that was absent to minimal (× 200). J. Tumor's peripheral vasoformative features (arrows) indicate anastomosing vascular channels lined by malignant endothelium (× 100). These marginal characteristics correspond to needle-like projections in periphery of nodule seen in CT image A. K-M. Immunohistochemistry staining shows tumor cells are diffusely positive for SMA (K), and CD31 (L), and weakly positive for Ki-67 (M) (× 200). SMA = smooth muscle actin
|
Table 1
Reported Cases of Pulmonary Angiosarcomas

References
1. Chen YB, Guo LC, Yang L, Feng W, Zhang XQ, Ling CH, et al. Angiosarcoma of the lung: 2 cases report and literature reviewed. Lung Cancer. 2010; 70:352–356.
2. Eichner R, Schwendy S, Liebl F, Huber A, Langer R. Two cases of primary pulmonary angiosarcoma as a rare cause of lung haemorrhage. Pathology. 2011; 43:386–389.
3. Patel AM, Ryu JH. Angiosarcoma in the lung. Chest. 1993; 103:1531–1535.
4. Weissferdt A, Moran CA. Primary vascular tumors of the lungs: a review. Ann Diagn Pathol. 2010; 14:296–308.
5. Jeong YJ, Lee KS, Jeong SY, Chung MJ, Shim SS, Kim H, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology. 2005; 237:675–683.
6. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010; 11:983–991.
7. Bastiaannet E, Groen H, Jager PL, Cobben DC, van der Graaf WT, Vaalburg W, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev. 2004; 30:83–101.
8. Eary JF, Conrad EU, Bruckner JD, Folpe A, Hunt KJ, Mankoff DA, et al. Quantitative [F-18] fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res. 1998; 4:1215–1220.
9. Tokmak E, Ozkan E, Yağcı S, Kır KM. F18-FDG PET/CT Scanning in Angiosarcoma: Report of Two Cases. Mol Imaging Radionucl Ther. 2011; 20:63–66.
10. Cucci E, Ciuffreda M, Tambaro R, Aquilani L, Barrassi M, Sallustio G. MRI findings of large low-grade angiosarcoma of the breast with subsequent bone metastases: a case report. J Breast Cancer. 2012; 15:255–257.
11. Lim RF, Goei R. Best cases from the AFIP: angiosarcoma of the breast. Radiographics. 2007; 27:Suppl 1. S125–S130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download