Abstract
Spinal dural arteriovenous fistula (SDAVF) is the most common spinal vascular malformation, however it is still rare and underdiagnosed. Magnetic resonance imaging findings such as spinal cord edema and dilated and tortuous perimedullary veins play a pivotal role in the confirmation of the diagnosis. However, spinal angiography remains the gold standard in the diagnosis of SDAVF. Classic angiographic findings of SDAVF are early filling of radicular veins, delayed venous return, and an extensive network of dilated perimedullary venous plexus. A series of angiograms of SDAVF at different locations along the spinal column, and mimics of serpentine perimedullary venous plexus on MR images, are demonstrated. Thorough knowledge of SDAVF aids correct diagnosis and prevents irreversible complications.
Spinal dural arteriovenous fistula (SDAVF) is the most common spinal vascular malformation, however it is still rare and frequently mis- or under-diagnosed. In the early stages, the clinical symptoms can be nonspecific and SDAVF is often initially misdiagnosed as sensory polyneuropathy, acute or chronic inflammatory demyelinating polyneuropathy, spinal muscular atrophy, or medullary tumor. Many patients even undergo surgery for lumbar disc herniation without an evident improvement in the clinical symptoms. If left untreated, the disease can progress to serious morbidity and cause irreversible handicap.
Due to nonspecific symptoms, the neuroradiologist is often the first clinician to make the initial diagnosis based on MR imaging, with differential diagnoses causing confusion. In addition, spinal angiography is the gold standard and should be taken into consideration where SDAVF is highly suspected. A neuroradiologist should interpret the spinal angiography carefully in order to differentiate between SDAVF and other possible mimics.
The clinical course of SDAVF is slow and progressive (1). Initially, the symptoms can be nonspecific such as tingling pain, weakness, gait disturbance, paresthesia, and diffuse or patchy sensory loss (234). Back pain without specific radicular distribution commonly occurs with a burning sensation or cramping (3). These neurological deficits progress and become worse with time (5). Acute deterioration sometimes presents after exercise, prolonged standing, changing position, and even singing and eating (6), which may be relieved after rest and mimic an anterior spinal artery syndrome (2). Intraspinal hemorrhage is very rare, however a unique case report described a patient with SDAVF at L4 suffering from spinal subarachnoid hemorrhage (7). Patients with a dural arteriovenous fistula at the craniocervical junction may present with acute headache due to intracranial subarachnoid hemorrhage (89101112). For the majority of patients, it takes on average 10-15 months before a diagnosis is made (24), and at diagnosis, bowel and bladder incontinence, erectile dysfunction, and urinary retention are almost always seen (24). Clinicians often use the Aminoff and Logue scale to evaluate the clinical symptoms, and to compare with post-treatment conditions (131314).
The exact etiology of SDAVF remains unclear. The majority of patients become symptomatic in middle age (15161718), suggesting that it is an acquired disease. Male predilection is also well known (18). Trauma is not considered a major causative factor in SDAVF due to the fact that trauma to the spine has been reported in merely 4% of patients (2). Furthermore, cervical SDAVF is rare despite the fact that the cervical spine is the most mobile segment of the spinal column and is more prone to trauma (218).
As compared with spinal arteriovenous malformation (AVM), SDAVF is never located within the spinal parenchyma and seldom causes intramedullary hemorrhage. It occurs in the cervical region much less frequently, and patients with SDAVF are older than those with spinal AVM (15).
Typically, one (sometimes multiple) feeding artery from a radiculopial artery or a dural branch of a radiculopial artery enters an intervertebral or radicular vein and forms a SDAVF within the dorsal surface of the dural root sleeve in the intervertebral foramen (Fig. 1) (5151617).
The feeding artery 'arterializes' the intervertebral vein, destroys the arteriovenous gradient and increases venous pressure, resulting in wall thickening and tortuosity of radial veins (also known as intramedullary veins), since intervertebral and radial veins share a common venous outflow (1920). Subsequently, chronic venous hypertension and stagnation decrease tissue perfusion and lead to edema of the spinal cord and progressive myelopathy. This may explain why the symptoms sometimes deteriorate after exercise or physical activities (6). The perimedullary venous plexus receives blood from radial veins draining the intramedullary circulation from the spinal cord dorsally in 80-90% of cases (21). Accumulated venous pressure can transmit through the perimedullary venous plexus in the caudo-cranial direction along the spinal cord (5). Extension can also go ventral and upwards to the cervical region or intracranial dural sinuses (22).
Radicular veins in the lower thoracic and lumbar region are fewer (23) and smaller in caliber, while venous drainage in the cervical region is divergent rather than convergent (1624). Therefore the lower thoracic and lumbar spinal cord is more vulnerable to hemodynamic changes, and the initial symptoms of SDAVF usually reflect dysfunction of the lower part of the spinal cord, that is, the conus medullaris (52526). Cervical SDAVF is rare, and presents with hemorrhage more often than thoracolumbar counterparts, possibly due to a higher blood flow rate. SDAVF is often located at the upper cervical level with arterial feeding from the right-sided vessels, particularly the vertebral artery, and frequently in association with complex coincidental vascular lesions (8).
Tadié et al. (23), demonstrated a narrowing of the intervertebral veins at the point crossing the dura mater. It loses its vascular wall that is replaced by an arachnoid cuff by the dura mater, showing a zigzag fashion when exiting the dura. It was hypothesized that the zigzag fashion and the narrowing of the intervertebral vein prevent blood flow going backwards to the intradural space, only permitting blood flow in a physiological direction. According to this observation, the "protective anti-flow back system" may be damaged in cases of SDAVF by congenital or acquired factors. However, patients with SDAVF may be asymptomatic despite the damaged "anti-flow back system" due to alternate venous outflow into ascending perimedullary veins, which open to the external paravertebral venous plexus via the upper cervical region. In these cases, SDAVF results in dilated perimedullary venous plexus but no venous hypertension or spinal cord edema (27). The average age of patients with asymptomatic SDAVF is roughly 10 years younger than that of patients with symptomatic SDAVF. Spontaneous angiographic conversion of progressive thrombosis and venous outflow obstruction related to aging has been reported in intracranial dural arteriovenous fistulae (28). It is noteworthy that the spontaneous angiographic conversion related to aging may also induce clinical symptoms in patients with asymptomatic SDAVF (27).
Myelography could be used as a screening examination prior to spinal angiography (29). Typical findings include the presence of prominent vessels extending over an average of eight levels, as well as beading of the cauda equine (30). Computed tomography angiography is also considered as an efficient method of localization with its high resolution and high contrast, reducing the time required for angiography (31). However, magnetic resonance imaging (MRI) and catheter angiography techniques are important in the confirmation of the diagnosis when there is a clinical suspicion of a progressive myelopathy.
The cord edema is most clearly shown as hyperintensities with peripheral sparing on T2-weighted images (Fig. 2A) (3032333435). It could extend over an average of 5-7 vertebrae, with conus involvement in over 80% of cases, which are usually homogeneous (3034). A serpentine and dilated intervertebral vein or engorged perimedullary venous plexus as flow voids are another striking characteristic on T2-weighted images (Fig. 2A). Nevertheless, if the arterialized veins are too small to visualize, contrast enhancement may play a pivotal role at diagnosis (Fig. 2B, C). MR angiography can be helpful in identifying the possible arterial feeders, and avoid unnecessary superselective injections of all possible arterial feeders in more than two-thirds of patients (3235). With a negative MRI scan and no findings resembling SDAVF on MR angiography, SDAVF is unlikely to be the culprit of the symptoms (303236).
Spinal catheter angiography, the gold standard in the diagnosis of SDAVF (18), should be considered if SDAVF is highly suspected due to its superior sensitivity compared with MR angiography. Ultimately, it is a treatable disease and severe morbidity can be avoided. The most frequent locations are the lower portion of the thoracic spine and the upper portion of the lumbar spine (23), and 70% of SDAVFs are located on the left side in the Toronto series (4). The potential feeding artery can be as cranial as the intracranial vessels and as caudal as the lateral sacral artery or inferior hypogastric artery (Figs. 3, 4, 5, 6) (37). Localization of these lesions may be difficult and challenging, especially in cases with cord edema distant from the arteriovenous shunt location.
During catheter angiography, the intercostal and lumbar arteries should be viewed as potential feeding arteries, in addition to the median and lateral sacral arteries and the deep cervical and ascending cervical arteries. Once an arterial feeder of SDAVF is localized, the tributaries at the ipsilateral and contralateral pedicle above and below the fistula should be catheterized to exclude the possibility of multiple feeding arteries to the fistula from the adjacent intercostal or lumbar arteries (29). Stasis of contrast material in the radiculomedullary arteries, especially the anterior spinal artery, can be found in catheter angiography followed by early filling of radicular veins and delayed venous return (527). An extensive network of dilated perimedullary venous plexus is usually also visible.
Spinal cord edema and serpentine perimedullary venous plexus are two main characteristics of a SDAVF on MRI. In terms of spinal cord edema, the central hyperintensity on T2-weighted images resembles findings of anterior spinal artery infarction, myelitis, spinal cord neoplasms (38), or a persistent central canal if slit-like (39). In comparison, spinal cord edema due to a SDAVF is usually homogenous with a tapered margin and extends across an average of 5-7 vertebrae with conus involvement (34).
Differential diagnoses for serpentine perimedullary venous plexus include flow voids on T2-weighted images caused by severe spinal canal stenosis and spinal extradural arteriovenous fistulas (SEAVF), and artifacts caused by turbulent flow of cerebrospinal fluid (CSF).
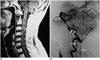
Severe spinal canal stenosis may display an engorged and tortuous vein, which is usually across only 1-2 vertebrae, along with a tangle of redundant nerves and vessels just proximal to the level of stenosis on myelography or MR myelography (Fig. 7). MRI helps to avoid confusion and shows no spinal cord edema or serpentine flow voids on the dorsal surface of the spinal cord.
Dural or epidural arterial branches drain primarily into epidural venous plexuses and form a SEAVF that is even rarer than SDAVF. To recognize and differentiate a SEAVF from a SDAVF is essential due to the fact that the ventral location to the dural sac and the arterialized venous lakes are challenging in hemostasis during surgery. The arterial feeders of SEAVF could originate from multiple levels, and the engorged epidural venous plexuses may cross the midline and fill multiple compartments, showing characteristic diamond-shaped plexuses (Fig. 8). A SDAVF, however, usually has a single feeding artery and drains intradural veins without filling the epidural space.
Turbulent flow of CSF moving from the ventral to the dorsal part of the subarachnoid space, combined with cardiac and respiratory related pulsatile CSF flow, leads to signal loss seen clearly on sagittal T2-weighted images, which is a frequently encountered artifact (40). It mostly occurs in children due to a relatively wider CSF space at the thoracic level, as well as patients with residual dorsal arachnoid septum posticum or spinal arachnoid cysts. The signal loss is bulky, discontinuous, and prone to be observed at the thoracic level (Fig. 9).
Spontaneous closure of a SDAVF without treatment is extremely rare (41). Treatment is essential because an untreated SDAVF can progress to serious morbidity and cause irreversible handicap.
Treatment options of a SDAVF include endovascular embolization and surgical ligation of the fistula. Endovascular embolization is less invasive. First of all, superselective catheterization of the feeding radiculopial artery should be carried out. The goal of treatment of SDAVF is to occlude the nidus as well as the first 1-2 cm of the proximal draining vein of the fistula (42). Embolization of only the distal artery may relieve the symptoms temporarily but almost always inevitably leads to recurrence due to numerous collaterals (43). Embolization with liquid polymers such as N-butyl 2-cyanoacrylate (NBCA), which is commonly referred to as glue, is recommended for a higher success rate of 44-100% (4444546). On the contrary, particles such as polyvinyl alcohol are thought to be related to the high recurrence rate of 30-93% (47) due to early recanalization, and coils and gelfoam are contraindicated for the same reason (5). The concentration of NBCA is a significant predictor of successful endovascular embolization. A higher concentration of NBCA presumably polymerizes faster and does not penetrate well. A lower concentration of NBCA polymerizes slower, but may not penetrate well either due to the higher viscosity resulting from the higher amount of iophendylate (lipiodol). The optimal concentration of NBCA is reported to be approximately 20-30% in order to minimize the obstacles of fast polymerization and high viscosity (48). In our experience, it is best accomplished with a mixture that has a long polymerization time such as NBCA and lipiodol in the ratio 1:4 (20%), which may be adjusted to 1:3 (25%) if the blood flow is fast. The mixture can be injected in a consecutive column or with a so-called sandwich or push technique. The direction of flow during final microcatheter injection is another determining factor in procedural success. When the flow is predominantly towards the fistula, the glue mixture has a higher chance of reaching the draining vein (48).
Ethylene vinyl alcohol (Onyx) is a liquid embolic material dissolved in dimethyl sulfoxide that polymerizes soon after contact with the bloodstream (49). Advantages of Onyx include lower viscosity, which facilitates venous penetration and decreases the risk of microcatheter retention after embolization, and theoretical permanent polymerization with empirical evidence of a low rate of recanalization of onyx-embolized aneurysms at the 5-year follow-up (50). Disadvantages of Onyx include the requirement for compatible catheters, higher expense, and the relative lack of availability in many centers (42). Transarterial Onyx embolization has been accepted as a promising therapeutic option for impressive feasibility and safety over NBCA, while long-term results remain controversial (425152).
Endovascular embolization is not always viable. If a spinal cord-supplying artery arises from the same pedicle as the feeder to the shunt, such as the great anterior radiculomedullary artery that is also known as the artery of Adamkiewicz, embolization may cause spinal cord ischemia (Fig. 12). Furthermore, technical barriers such as severe atherosclerosis, arterial dissection of the feeding arteries, or extensive collateral vessels may halt superselection of target vessels.
Surgical treatment should be considered if endovascular embolization fails. Currently, ligation of the draining vein is recommended (53), and has been proven to be as effective as total removal of the draining vein (54).
The success rate of surgery (98%) is higher than that of embolization (46%) (55). However, embolization is less invasive and more efficient when only a single arterial feeder is shown, and can be used preoperatively to label the feeding artery (5). Generally, the prognosis depends on the duration of symptoms, pretreatment disabilities, and degrees of obliteration of the fistula and draining veins, however, no reliable prognostic factors have yet been identified (45565758).
It has been reported that 55% of patients had improved gait after microsurgery, 11% deteriorated, and the rest remained stationary, with only 33% having improvement in micturition. These results are compatible with vascular anatomy and the distribution of motor and sensory pathways in the spine. Other studies have also yielded a similar conclusion that patients benefit most from the treatment, generally endovascular embolization, in motor and pain symptoms, followed by sensory disturbance, and sphincter and erectile dysfunctions (5658596061). It is reasonable to presume that endovascular embolization could be as effective in patients with complete obliteration of the fistula as in those who undergo surgery.
If there is no improvement within 4-6 weeks following treatment, or even clinical deterioration during follow-ups, repeat MRI should be performed. Once MRI distinctly demonstrates persistent flow voids along the spinal cord in a patient with a poor response to the previous treatment, repeat angiography is indicated (62). Residual fistula caused by inadequate occlusion or recanalization, and rarely but not impossibly a second fistula, should be looked for carefully. Therefore, follow-up angiography is recommended to be carried out 2-3 months after surgical or endovascular treatment, due to the fact that clinical improvement starts to reach a plateau around that time. High suspicion of residual fistula prompts an earlier follow-up angiography (63).
Spinal dural arteriovenous fistula is rare but frequently underdiagnosed since the clinical presentations resemble other more common causes of myelopathy. The disease is treatable but can progress to serious morbidity and irreversible damage. That is, the earlier the diagnosis, the better the outcome. MR imaging and spinal angiography should be considered under high clinical suspicion. Subsequently, prompt endovascular embolization or surgical ligation should be performed according to the conditions of the patient and characteristics of the fistula. Thorough knowledge regarding SDAVF may enable clinicians and neuroradiologists to diagnose the disease in the early stages.
Figures and Tables
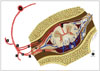 | Fig. 1Illustration of SDAVF at lower thoracic level.Arteries are labeled with black background, while veins are labeled with white background. a = aorta, b = intercostal artery, c = dorsal branch of spinal artery, d = ventral branch of spinal artery, e = ventral epidural arcade, f = dural branch of ventral spinal artery, g = radicular artery, h = radiculomedullary artery, i = radiculopial artery, j = anterior spinal artery, k = dural branches of radiculopial artery, l = radial vein, m = perimedullary venous plexus, n = posterior radicular vein, o = anterior radicular vein, p = intervertebral vein, q = epidural venous plexus.
|
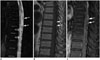 | Fig. 2Characteristic diagnostic clues of SDAVF on MRI.
A. On sagittal T2-weighted spinal MR image, spinal cord hyperintensity (hollow arrows), and serpentine and dilated perimedullary venous plexus (white arrows) as flow voids are striking characteristics. B. On sagittal T1-weighted spinal MR image, tortuous and dilated perimedullary venous plexus is barely recognized (white arrows). C. Sagittal T1-weighted MR image with gadolinium enhancement clearly reveals tortuous and dilated perimedullary venous plexus (white arrows). SDAVF = spinal dural arteriovenous fistula
|
 | Fig. 3Intracranial dural arteriovenous fistula with arterial feeding from meningeal branch of left vertebral artery of 47-year-old man with progressive quadriparesis associated with urinary and fecal incontinence and impotency.
A. Sagittal T1-weighted spinal MR image with gadolinium enhancement shows serpentine enhancement at anterior and posterior surfaces of cervical spinal cord and brainstem, as well as at tonsils (arrowheads). B. Lateral view of left vertebral arteriogram demonstrates engorged inferior vermian vein (white arrows), which connects to veins at lower brainstem to drain into anterior and posterior spinal veins (hallow arrows). Reproduced with permission from Chen et al. Neuroradiology 1998;40:393-397 (64).
|
 | Fig. 4Cervical SDAVF with feeding artery from right vertebral artery of 84-year-old man as incidental finding.
A. Right verterbral angiogram in frontal view at early phase demonstrates suspicious SDAVF (white arrows) with feeding artery directly from right vertebral artery (hallow arrow). B. Dilated and tortuous draining vein is prominent at late phase (black arrows). SDAVF = spinal dural arteriovenous fistula
|
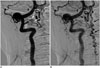
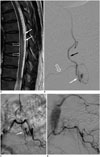 | Fig. 5Lumbar SDAVF with arterial feeding from left L2 lumbar artery of 53-year-old man presenting with bilateral weakness in lower extremities and urinary bladder frequency.
A. Sagittal T2-weighted spinal MR image reveals hyperintensity (hollow arrows) with flow voids (white arrows) mostly at posterior surface of spinal cord. B. Superselective angiography evidently shows feeding artery (hallow arrow), SDAVF (white arrow) and tortuous draining vein (black arrow). SDAVF = spinal dural arteriovenous fistula C. Angiogram of left L2 lumbar artery in frontal view before treatment demonstrates fistula (white arrow) and tortuous vein (hollow arrow). D. Angiogram of left L2 lumbar artery in frontal view after treatment reveals result of successful endovascular embolization without opacification of fistula or tortuous vein.
|
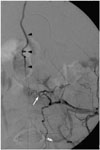 | Fig. 6Sacral SDAVF with arterial feeding from left internal iliac artery of 67-year-old male suffering from progressive numbness, soreness and muscle cramps in bilateral lower extremities for 2 years.Left internal iliac angiogram in frontal view shows sacral SDAVF (white arrow) draining toward spinal canal via dilated filum terminale vein (arrowheads). Reproduced with permission from Chen et al. Eur J Radiol 2002;44:152-155 (65). SDAVF = spinal dural arteriovenous fistula
|
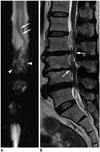 | Fig. 776-year-old man with lower legs numbness was diagnosed with severe canal stenosis at L3/4 and L4/5 and moderate canal stenosis at L2/3.
A. MR myelography displays engorged and tortuous vein (white arrows) across only about 2 vertebrae along with tangle of flow voids (arrowheads), which are possibly redundant nerves and vessels, just proximal to level of stenosis. These findings are distinguishable from that of Figure 2A. B. Sagittal T2-weighted spinal MR image shows annular bulging at L2/3 to L4/5, causing moderate and severe spinal canal stenosis (white arrows) without spinal cord edema.
|
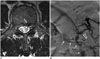 | Fig. 860-year-old man presenting with bilateral leg soreness for several months was diagnosed with SEAVF.
A. Axial T2-weighted spinal MR image shows engorged flow void structure (white arrow) at left anterolateral epidural space at L3 level, which is probably epidural venous lake. B. Angiography of left lumbar artery at L3 shows arteriovenous shunts (hollow arrow) that drain into prominent, diamond-shaped epidural venous plexus (white arrows) cross midline and delayed intradural venous drainage (black long arrows). Turning point (small black arrows) where retrograde drainage of intradural vein arises from epidural venous plexus is clearly exhibited. SEAVF = spinal extradural arteriovenous fistulas
|
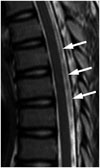 | Fig. 913-year-old girl presented with nonspecific intermittent back pain.No definite diagnosis was established. Sagittal T2-weighted spinal MR image shows bulky and discontinuous signal loss (white arrows) at thoracic level without spinal cord edema.
|
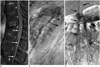 | Fig. 10Prominent anterior spinal artery mimics SDAVF.
A. Sagittal gadolinium-enhanced MR image demonstrates prominent vessels on anterior surface of spinal cord (white arrows). B, C. Lateral (B) and frontal (C) views of left T12 intercostal arteriogram reveal prominent artery in middle of anterior surface of spinal cord (black arrows) with characteristic hairpin turn, which is anterior spinal artery supplying from artery of Adamkiewicz. No SDAVF was found based on this study. SDAVF = spinal dural arteriovenous fistula
|
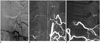 | Fig. 11Prominent left posterior spinal artery mimics SDAVF.
A. Left L1 lumbar arteriogram shows longitudinal artery without hairpin turn. B, C. On coronal (B) and sagittal (C) three-dimensional CT angiograms, artery is paramedian and dorsal to spinal cord (hollow arrow), proven to be prominent left posterior spinal artery. White dotted line demonstrates midline. SDAVF = spinal dural arteriovenous fistula
|
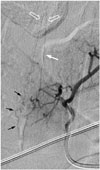 | Fig. 12SDAVF with arterial feeding from artery of Adamkiewicz of 29-year-old woman presenting with progressive tingling and paresthesia in lower extremities for 6 months.T5 intercoastal arteriogram at early phase demonstrates that artery of Adamkiewicz (white arrow) ascends first then joins anterior spinal artery after characteristic hairpin turn (hollow arrows). Simultaneous appearance of serpentine and tortuous perimedullary venous plexus (black arrows) implies common origin with artery of Adamkiewicz. SDAVF = spinal dural arteriovenous fistula
|
References
1. Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974; 97:211–218.
2. Jellema K, Canta LR, Tijssen CC, van Rooij WJ, Koudstaal PJ, van Gijn J. Spinal dural arteriovenous fistulas: clinical features in 80 patients. J Neurol Neurosurg Psychiatry. 2003; 74:1438–1440.
3. Koch C. Spinal dural arteriovenous fistula. Curr Opin Neurol. 2006; 19:69–75.
4. Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke. 2002; 33:1578–1583.
5. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009; 30:639–648.
6. Khurana VG, Perez-Terzic CM, Petersen RC, Krauss WE. Singing paraplegia: a distinctive manifestation of a spinal dural arteriovenous fistula. Neurology. 2002; 58:1279–1281.
7. Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004; 100:4 Suppl Spine. 385–391.
8. Kim DJ, Willinsky R, Geibprasert S, Krings T, Wallace C, Gentili F, et al. Angiographic characteristics and treatment of cervical spinal dural arteriovenous shunts. AJNR Am J Neuroradiol. 2010; 31:1512–1515.
9. Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998; 89:755–761.
10. Do HM, Jensen ME, Cloft HJ, Kallmes DF, Dion JE. Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol. 1999; 20:348–350.
11. Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci. 2000; 7:64–66.
12. Kim MS, Han DH, Han MH, Oh CW. Posterior fossa hemorrhage caused by dural arteriovenous fistula: case reports. Surg Neurol. 2003; 59:512–516. discussion 516-517
13. Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008; 62:159–166. discussion 166-167
14. Atkinson JL, Miller GM, Krauss WE, Marsh WR, Piepgras DG, Atkinson PP, et al. Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin Proc. 2001; 76:1120–1130.
15. Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM's in 81 patients. J Neurosurg. 1987; 67:795–802.
16. Berenstein A, Lasjaunias P, ter Brugge KG. Spinal dural arteriovenous fistulae. In : Berenstein A, Lasjaunias P, ter Brugge KG, editors. Surgical neuroangiography. 2nd ed. Berlin: Springer;2004. p. 849–872.
17. Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg. 1984; 60:238–247.
18. Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006; 129(Pt 12):3150–3164.
19. Kataoka H, Miyamoto S, Nagata I, Ueba T, Hashimoto N. Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery. 2001; 48:1224–1229. discussion 1229-1230
20. Hurst RW, Kenyon LC, Lavi E, Raps EC, Marcotte P. Spinal dural arteriovenous fistula: the pathology of venous hypertensive myelopathy. Neurology. 1995; 45:1309–1313.
21. Anson JA, Spetzler RF. Endarterectomy of the intradural vertebral artery via the far lateral approach. Neurosurgery. 1993; 33:804–810. discussion 810-811
22. Koenig E, Thron A, Schrader V, Dichgans J. Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol. 1989; 236:260–266.
23. Tadié M, Hemet J, Freger P, Clavier E, Creissard P. Morphological and functional anatomy of spinal cord veins. J Neuroradiol. 1985; 12:3–20.
24. Moss JG, Sellar RJ, Hadley DM. Intracerebral and spinal vascular malformation in a patient without hereditary haemorrhagic telangiectasia. Neuroradiology. 1989; 31:280–281.
25. Vasdev A, Lefournier V, Bessou P, Dematteis M, Crouzet G. [Intracranial dural fistula with spinal cord venous drainage. Apropos of 2 cases]. J Neuroradiol. 1994; 21:134–154.
26. Asakawa H, Yanaka K, Fujita K, Marushima A, Anno I, Nose T. Intracranial dural arteriovenous fistula showing diffuse MR enhancement of the spinal cord: case report and review of the literature. Surg Neurol. 2002; 58:251–257.
27. Sato K, Terbrugge KG, Krings T. Asymptomatic spinal dural arteriovenous fistulas: pathomechanical considerations. J Neurosurg Spine. 2012; 16:441–446.
28. Kim DJ, terBrugge K, Krings T, Willinsky R, Wallace C. Spontaneous angiographic conversion of intracranial dural arteriovenous shunt: long-term follow-up in nontreated patients. Stroke. 2010; 41:1489–1494.
29. Chen CJ, Ro LS, Cheng WC, Chen ST. MRI/myelographic localization of fistulous tract in spinal dural arteriovenous malformations prior to arteriography. J Comput Assist Tomogr. 1995; 19:893–896.
30. Gilbertson JR, Miller GM, Goldman MS, Marsh WR. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol. 1995; 16:2049–2057.
31. Lai PH, Weng MJ, Lee KW, Pan HB. Multidetector CT angiography in diagnosing type I and type IVA spinal vascular malformations. AJNR Am J Neuroradiol. 2006; 27:813–817.
32. Bowen BC, Fraser K, Kochan JP, Pattany PM, Green BA, Quencer RM. Spinal dural arteriovenous fistulas: evaluation with MR angiography. AJNR Am J Neuroradiol. 1995; 16:2029–2043.
33. Jones BV, Ernst RJ, Tomsick TA, Tew J Jr. Spinal dural arteriovenous fistulas: recognizing the spectrum of magnetic resonance imaging findings. J Spinal Cord Med. 1997; 20:43–48.
34. Hurst RW, Grossman RI. Peripheral spinal cord hypointensity on T2-weighted MR images: a reliable imaging sign of venous hypertensive myelopathy. AJNR Am J Neuroradiol. 2000; 21:781–786.
35. Luetmer PH, Lane JI, Gilbertson JR, Bernstein MA, Huston J 3rd, Atkinson JL. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR Angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005; 26:711–718.
36. Saraf-Lavi E, Bowen BC, Quencer RM, Sklar EM, Holz A, Falcone S, et al. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol. 2002; 23:858–867.
37. Macht S, Chapot R, Bieniek F, Hänggi D, Turowski B. Unique sacral location of an arteriovenous fistula of the filum terminale associated with diastematomyelia and lowered spinal cords. Neuroradiology. 2012; 54:517–519.
38. Grandin C, Duprez T, Stroobandt G, Laterre EC, Mathurin P. Spinal dural arterio-venous fistula: an underdiagnosed disease? Acta Neurol Belg. 1997; 97:17–21.
39. Holly LT, Batzdorf U. Slitlike syrinx cavities: a persistent central canal. J Neurosurg. 2002; 97:2 Suppl. 161–165.
40. Lisanti C, Carlin C, Banks KP, Wang D. Normal MRI appearance and motion-related phenomena of CSF. AJR Am J Roentgenol. 2007; 188:716–725.
41. Meder JF, Devaux B, Merland JJ, Frédy D. Spontaneous disappearance of a spinal dural arteriovenous fistula. AJNR Am J Neuroradiol. 1995; 16:2058–2062.
42. Warakaulle DR, Aviv RI, Niemann D, Molyneux AJ, Byrne JV, Teddy P. Embolisation of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003; 45:110–112.
43. McCutcheon IE, Doppman JL, Oldfield EH. Microvascular anatomy of dural arteriovenous abnormalities of the spine: a microangiographic study. J Neurosurg. 1996; 84:215–220.
44. Hall WA, Oldfield EH, Doppman JL. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989; 70:714–720.
45. Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997; 40:675–682. discussion 682-683
46. Westphal M, Koch C. Management of spinal dural arteriovenous fistulae using an interdisciplinary neuroradiological/neurosurgical approach: experience with 47 cases. Neurosurgery. 1999; 45:451–457. discussion 457-458
47. Nichols DA, Rufenacht DA, Jack CR Jr, Forbes GS. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: experience in 14 patients. AJNR Am J Neuroradiol. 1992; 13:933–940.
48. Su IC, terBrugge KG, Willinsky RA, Krings T. Factors determining the success of endovascular treatments among patients with spinal dural arteriovenous fistulas. Neuroradiology. 2013; 55:1389–1395.
49. Carlson AP, Taylor CL, Yonas H. Treatment of dural arteriovenous fistula using ethylene vinyl alcohol (onyx) arterial embolization as the primary modality: short-term results. J Neurosurg. 2007; 107:1120–1125.
50. Cekirge HS, Saatci I, Ozturk MH, Cil B, Arat A, Mawad M, et al. Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology. 2006; 48:113–126.
51. Adamczyk P, Amar AP, Mack WJ, Larsen DW. Recurrence of "cured" dural arteriovenous fistulas after Onyx embolization. Neurosurg Focus. 2012; 32:E12.
52. Nogueira RG, Dabus G, Rabinov JD, Ogilvy CS, Hirsch JA, Pryor JC. Onyx embolization for the treatment of spinal dural arteriovenous fistulae: initial experience with long-term follow-up. Technical case report. Neurosurgery. 2009; 64:E197–E198. discussion E198
53. Huffmann BC, Gilsbach JM, Thron A. Spinal dural arteriovenous fistulas: a plea for neurosurgical treatment. Acta Neurochir (Wien). 1995; 135:44–51.
54. Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995; 82:196–200.
55. Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004; 55:77–87. discussion 87-88
56. Eskandar EN, Borges LF, Budzik RF Jr, Putman CM, Ogilvy CS. Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg. 2002; 96:2 Suppl. 162–167.
57. Cenzato M, Versari P, Righi C, Simionato F, Casali C, Giovanelli M. Spinal dural arteriovenous fistulae: analysis of outcome in relation to pretreatment indicators. Neurosurgery. 2004; 55:815–822. discussion 822-823
58. Jellema K, Tijssen CC, van Rooij WJ, Sluzewski M, Koudstaal PJ, Algra A, et al. Spinal dural arteriovenous fistulas: long-term follow-up of 44 treated patients. Neurology. 2004; 62:1839–1841.
59. Lundqvist C, Berthelsen B, Sullivan M, Svendsen P, Andersen O. Spinal arteriovenous malformations: neurological aspects and results of embolization. Acta Neurol Scand. 1990; 82:51–58.
60. Song JK, Vinuela F, Gobin YP, Duckwiler GR, Murayama Y, Kureshi I, et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg. 2001; 94:2 Suppl. 199–204.
61. Guillevin R, Vallee JN, Cormier E, Lo D, Dormont D, Chiras J. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae: CT evaluation, technical features, and outcome prognosis in 26 cases. AJNR Am J Neuroradiol. 2005; 26:929–935.
62. Mascalchi M, Ferrito G, Quilici N, Mangiafico S, Cosottini M, Cellerini M, et al. Spinal vascular malformations: MR angiography after treatment. Radiology. 2001; 219:346–353.
63. Willinsky RA, terBrugge K, Montanera W, Mikulis D, Wallace MC. Posttreatment MR findings in spinal dural arteriovenous malformations. AJNR Am J Neuroradiol. 1995; 16:2063–2071.
64. Chen CJ, Chen CM, Lin TK. Enhanced cervical MRI in identifying intracranial dural arteriovenous fistulae with spinal perimedullary venous drainage. Neuroradiology. 1998; 40:393–397.
65. Chen CJ, Hsu HL. Engorged and tortuous intradural filum terminale vein as a sign of a sacral dural arteriovenous malformation. Eur J Radiol. 2002; 44:152–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download