Abstract
Portal vein embolization (PVE) is known as an effective and safe preoperative procedure that increases the future liver remnant (FLR) in patients with insufficient FLR. However, some possible major complications can lead to non-resectability or delayed elective surgery that results in increased morbidity and mortality. Although the majority of these complications are rare, knowledge of the radiologic findings of post-procedural complications facilitate an accurate diagnosis and ensure prompt management. We accordingly reviewed the CT findings of the complications of PVE.
A smaller "future liver remnant (FLR)" after extensive hepatic resection is associated with increased mortality and morbidity. Portal vein embolization (PVE) has been performed in patients with smaller than desired FLR (12). This procedure induces hypertrophy of the remnant liver by selective embolization of a portion of the portal vein in the diseased liver (3).
Portal vein embolization is a relatively safe procedure and most patients experience no significant procedure-related complications (4). Few studies to date have focused on the major complications that can lead to nonresectability. In this study, we focused on the radiologic findings of the major complications after PVE.
Di Stefano et al. (5) reported 12.8% adverse events in patients who had PVE with a contralateral approach. Complications included complete portal vein thrombosis, inadvertent n-butyl-2-cyanoacrylate (NBCA) migration into the main portal vein feeding the FLR, hemoperitoneum, hemobilia, rupture of the metastasis, and transient hepatic failure (5). Incidental findings included subcapsular hematoma located at the puncture site and migration of small NBCA fragments into the FLR (5). van Lienden et al. (6) reported major complications with resulting nonresectability after PVE in 0.4% of their study population. These complications consisted of severe cholangitis, large abscesses and sepsis, and portal venous or mesentericoportal venous thrombosis.
We categorized major complications that can be evaluated on CT into puncture-related, i.e., vascular injury, biloma, and abscess, and embolization-related, i.e., migration of embolic material, parenchymal infarction, non-targeted embolization, and proximal venous thrombosis. We presented the CT findings of these major complications together with their clinical significance and treatment options.
Hemorrhage and other vascular injuries, including arteriovenous fistula, pseudoaneurysm, and transient hemobilia, are related to the puncture. Hemorrhage is the most common complication of percutaneous transhepatic procedures and reportedly occurs in approximately 2-4% of patients following PVE (7). It may present as subcapsular hematoma (Fig. 1) or hemoperitoneum (Fig. 2) that occurs immediately or in a delayed manner. For this reason, internal hemorrhage should be a concern when low blood pressure is noted even several weeks after the procedure.
There are several bleeding sources. These include the intercostal artery, portal vein, hepatic vein, and the hepatic artery. The bleeding site is usually along the tract of the guiding catheter. In their published studies, some clinicians recommend embolization of the puncture tract when retrieving the catheter (8). When there is massive bleeding, transarterial embolization can be the most effective treatment. The coagulation parameters should also be checked before PVE in order to prevent bleeding, especially in patients with chronic liver disease.
Biloma that develops after PVE appears to be associated with either intra- or extrahepatic bile duct injury during transhepatic puncture. The CT numbers of the biloma fluid are less than 20 Hounsfield units (Fig. 3), but may be higher when the bile is mixed with blood or exudates. Biloma can be confirmed by sonographic guidance needle aspiration and treated successfully by percutaneous catheter drainage. In cases of infected biloma that are non-responsive to percutaneous drainage and antibiotics administration, surgical drainage should be considered. Bile leak can also cause bile peritonitis without biloma formation. In such cases, patients may present with severe whole abdominal pain.
Infection may occur after a transhepatic procedure and may present as cholangitis, liver abscess (Fig. 4) or systemic sepsis. Many patients with hepatic abscess show rim enhancement and occasionally a honeycomb-like appearance. Perilesional hyperemic change can also appear in the adjacent hepatic parenchyma. The presence of preoperative infection, including cholangitis, has a negative impact not only on the regeneration capacity after PVE but also on the outcomes after a major hepatectomy (9).
Complications related to the embolization itself include embolic material migration, non-targeted vein embolization, proximal venous thrombosis, parenchymal infarction, and portal hypertension.
Follow-up study showing ectopic location of the embolic material is indicative of embolic material migration from the material located in the targeted vessel at the time of the procedure (Fig. 5). Amplatzer Vascular Plug (AVP, AGA Medical Corp., Golden Valley, MN, USA) is a self-expandable, cylindrical, nitinol wire mesh with lower migration risk than coils. Migration of the AVP is very rare even for embolization of arteries that are high-flow vessels (10). For this reason it has been used as a mechanical anchor to prevent other embolic material migration and to interrupt intra-procedural blood flow (10).
However, AVP migration can occur, possibly due to inappropriate size of the AVP, as compared to the targeted portal vein. Accurate measures of the target vessel diameter and selection of a suitable AVP that is 30-50% larger than the target vessel diameter is important to prevent AVP migration during PVE (11).
Thrombosis of the affected portal vein caused by the migrated embolic materials that does not affect a patient's hepatic function requires no further management (Fig. 5). However, portoportal graft during hepatic resection has been reported as treatment of portal vein occlusion secondary to embolic material migration (4).
Significant hemodynamic changes can occur after PVE and may manifest as the spectrum of differential hepatic parenchymal enhancement and parenchymal infarction (Fig. 6) (7). Portal vein occlusion induces a significant decrease in the hepatic resistive index in both healthy and diseased livers. This is indicative of increased hepatic arterial flow as a compensatory mechanism for the diminished portal flow. Therefore, systemic hypotension may lead to parenchymal infarction after PVE.
Hepatic infarction shows a varied appearance on CT scans. Round or oval, non-enhancing areas or relatively well-demarcated, wedge-shaped, low-attenuation lesions extending to the liver surface can be seen. The presence of non-displaced vessels coursing through areas of hepatic infarction excludes the possibility of space-occupying lesions such as abscess or tumor (12). Sometimes, biloma formation can be caused by bile duct necrosis. All these factors might affect the hepatic function as well as a patient's general condition, and can possibly lead to delayed, appropriate surgery or non-resectability.
Non-targeted embolization refers to inadvertent intra-procedural embolization in the FLR (Fig. 7). It is related to overflow or dislodgement of embolic materials by the catheter into the non-targeted liver when flow in the targeted portal vein branches was near stasis. A previous study on NBCA for PVE (5) indicated that migration of emboli is common; 2 of 188 patients with migration of embolic materials into the left portal vein feeding the FLR required portal vein angioplasty and 10 cases of small emboli in non-targeted portal branches were reported as incidental findings.
Non-targeted embolization is related to the kind of embolic materials used. Liquid embolic materials can flow more distally and can completely embolize the distal small branches of the portal vein (5). This means that recanalization occurs less often when using particle embolic materials or coils. However, liquid embolic materials may increase the risk of non-targeted embolization, especially for inexperienced operators. Liquid embolic materials cannot be removed, hence, the extent of the hepatic resection can be increased or liver function can be deteriorated.
Even though successful PVE has been performed, venous thrombosis can occur in the proximal or contralateral portal vein (Fig. 8). It is a rare though potentially major complication of PVE because acute fulminant liver failure or death can ensue. In the setting of PVE, precipitating factors of venous thrombosis include injury to the portal venous wall, reduced portal flow, portal hypertension, hypercoagulopathy, inflammatory process, malignancy, pregnancy, oral contraceptive use, and asplenia (13). Thrombosis of the non-targeted portal veins can be due to the formation or propagation of thrombus along the catheter shaft or venous occlusion by the catheter itself, in patients in whom the contralateral transhepatic approach is used (13). Di Stefano et al. (5) reviewed 188 patients who underwent PVE using a contralateral approach and reported 1 case of complete portal vein thrombosis. Anatomic variation can also contribute to left portal vein thrombosis. For example, when the embolized right anterior portal vein arises from the left portal vein, there is a potential increased risk of left portal vein thrombosis (Fig. 8).
Venous thrombosis is the worst possible complication because hypertrophy of the FLR may be compromised (5). It also requires immediate treatment. If a thrombus is acute or subacute and extensive, i.e., within either the main or left portal vein, thrombolysis with or without mechanical thrombectomy should be considered (13).
There usually are no clinical consequences when the rise in portal venous pressure is moderate (generally 5 to 7 mm Hg) after PVE (14). However, in cirrhotic patients with portal hypertension, this hemodynamic alteration can increase the risk of rupture of gastroesophageal varices.
Some possible major complications after PVE can lead to non-resectability or delayed elective surgery and can thus result in increased patient morbidity and mortality. Although the majority of these complications are rare, familiarity with the radiologic findings of post-procedural complications facilitate an accurate diagnosis and prompt management in these situations.
Figures and Tables
Fig. 1
73-year-old man with hilar cholangiocarcinoma.
Right portal vein embolization was aborted due to lack of left portal venous flow seen on direct portogram using right portal vein approach (not shown). A, B. Enhanced axial (A) and coronal (B) CT scans obtained 3 weeks later show large subcapsular hematoma at inferior aspect of liver segment VI. Note coils (arrow in B) used for tract embolization, adjacent to hematoma.
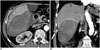
Fig. 2
50-year-old man with liver metastasis from colon cancer.
Low blood pressure progressed after left portal vein embolization. Enhanced coronal CT scans show large amount of hemoperitoneum. Left portal vein was embolized using coils (arrow).
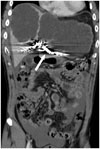
Fig. 3
77-year-old man with hilar cholangiocarcinoma.
Ten days after right portal vein embolization, patient came to emergency room due to severe, right upper quadrant pain. A, B. Enhanced axial (A) and coronal (B) CT scans show large subcapsular water attenuation fluid collection with mass effect on right hepatic lobe suggestive of biloma. Bile was confirmed after drainage tube insertion.
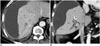
Fig. 4
64-year-old man with hilar cholangiocarcinoma.
Follow-up enhanced axial CT scan obtained 2 weeks after right portal vein embolization shows multi-lobulated, low-attenuation lesions, suggesting abscess, in liver segment VI. Elective right hepatectomy was performed after antibiotics administration for one week.
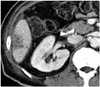
Fig. 5
77-year-old woman with cholangiocarcinoma.
A. Right portal vein embolization was successfully performed with Amplatzer Vascular Plug (AVP) (black arrow, 10-mm in diameter and 7-mm in length) and gelatin sponge particles. AVP is located close to portal confluence. B, C. Enhanced axial (B) and coronal (C) CT scans obtained 1 week after right hemihepatectomy, show migration of AVP to left portal vein. Partial thrombosis distal to migrated AVP is observed in umbilical segment of left portal vein (white arrow). Liver function was not deteriorated. D. Enhanced axial CT scan taken 4 years later shows no change of migrated AVP in left portal vein and disappearance of distal partial thrombosis.
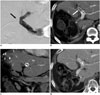
Fig. 6
67-year-old man with hepatocellular carcinoma.
One day after portal vein embolization, his liver enzymes had markedly elevated. Portal-phase axial CT scan shows decreased enhancement (arrows) in right hepatic lobe and small air densities along right portal vein and liver parenchyma suggestive of parenchymal infarction.
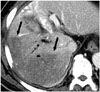
Fig. 7
72-year-old woman with hilarcholangiocarcinoma.
A. Initial portogram shows trifurcation of right anterior and posterior portal veins and left portal veins. B. During right anterior and posterior portal vein embolization (PVE) with n-butyl-2-cyanoacrylate (NBCA) and gelatin sponge particles, unintended left PVE occurred (black arrow). C. Follow-up enhanced axial CT scan shows some NBCA cast in left portal vein (black arrow). Patient was not able to undergo surgery due to hepatic dysfunction.
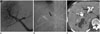
Fig. 8
50-year-old man with hepatocellular carcinoma.
A. Initial portogram shows right anterior portal vein (white arrows) arising from left portal vein. B. Right anterior portal vein was embolized with coils and gelatin sponge particles. C. Portal phases of enhanced CT scan obtained 3 weeks after portal vein embolization (PVE), show partial thrombosis (black arrow) in left portal vein. One month after PVE, left trisegmentectomy was performed because liver function test had not deteriorated.

References
1. Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg. 2013; 100:1777–1783.
2. May BJ, Talenfeld AD, Madoff DC. Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol. 2013; 24:241–254.
3. Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000; 232:665–672.
4. May BJ, Madoff DC. Portal vein embolization: rationale, technique, and current application. Semin Intervent Radiol. 2012; 29:81–89.
5. Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005; 234:625–630.
6. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013; 36:25–34.
7. Ganeshan DM, Szklaruk J. Portal vein embolization: cross-sectional imaging of normal features and complications. AJR Am J Roentgenol. 2012; 199:1275–1282.
8. Park SY, Kim J, Kim BW, Wang HJ, Kim SS, Cheong JY, et al. Embolization of percutaneous transhepatic portal venous access tract with N-butyl cyanoacrylate. Br J Radiol. 2014; 87:20140347.
9. Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery. 2014; 156:1190–1196.
10. Cho YK, Chang NK, Ma JS. Successful transcatheter closure of a large patent ductus venosus with the Amplatzer vascular plug II. Pediatr Cardiol. 2009; 30:540–542.
11. Ratnam LA, Walkden RM, Munneke GJ, Morgan RA, Belli AM. The Amplatzer vascular plug for large vessel occlusion in the endovascular management of aneurysms. Eur Radiol. 2008; 18:2006–2012.
12. Holbert BL, Baron RL, Dodd GD 3rd. Hepatic infarction caused by arterial insufficiency: spectrum and evolution of CT findings. AJR Am J Roentgenol. 1996; 166:815–820.
13. Shaw CM, Madoff DC. Acute Thrombosis of Left Portal Vein during Right Portal Vein Embolization Extended to Segment 4. Semin Intervent Radiol. 2011; 28:156–161.
14. Huang JY, Yang WZ, Li JJ, Jiang N, Zheng QB. Portal vein embolization induces compensatory hypertrophy of remnant liver. World J Gastroenterol. 2006; 12:408–414.
15. Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006; 23:126–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download