Abstract
Immunoglobulin G4 (IgG4)-related kidney disease (IgG4-KD) has recently been demonstrated to be an important part of IgG4-related sclerosing disease (IgG4-SD). However, since IgG4-KD is still relatively unfamiliar to radiologists and physicians as compared to IgG4-SD involving other organs, it could, therefore, be easily missed. In this article, we present a comprehensive pictorial review of IgG4-KD with regards to the imaging spectrum, mimickers, and clinicopathologic characteristics, based on our clinical experience with 48 patients during the past 13 years, as well as a literature review. Awareness of the broad imaging spectrum of IgG4-KD and differential diagnosis from its mimickers will thus facilitate its early diagnosis and treatment.
Immunoglobulin G4 (IgG4)-related kidney disease (IgG4-KD) is a comprehensive term indicating the renal involvement of IgG4-related sclerosing disease (IgG4-SD), which is an immune-mediated systemic disease that can affect almost every organ system (1). The most common feature of IgG4-KD is tubulointerstitial nephritis with increased IgG4-positive plasma cells, along with fibrosis (123456). IgG4-KD usually responds well to steroid therapy; however, if not properly treated, it may cause acute or chronic renal dysfunction and can progress to irreversible renal failure (6). Therefore, a timely and accurate diagnosis that ensures early steroid treatment is crucial for improving the patient outcome. In addition, as IgG4-KD is usually associated with IgG4-SD involving other organs, including autoimmune pancreatitis (AIP) or IgG4-related sclerosing cholangitis, identifying the renal lesions can provide clues for diagnosing IgG4-SD (56789). Therefore, radiologists must be aware of the imaging features of IgG4-KD, as well as also be familiar with this disease entity. The purpose of this article is to provide a comprehensive pictorial review of the imaging spectrum of IgG4-KD, and to discuss differential diagnosis from its mimickers. We will also briefly review the clinicopathologic characteristics of IgG4-KD.
Immunoglobulin G4-related kidney disease usually affects middle-aged or elderly patients and shows a definite male predominance (73-90%), which is similar in patients with IgG4-SD involving other organs (57910). IgG4-KD is usually accompanied by other organ involvement in IgG4-SD, typically AIP, and approximately 1/4 or 1/3 of AIP patients have IgG4-KD (111213). Isolated cases of IgG4-KD without other organ involvement, is very rare. In our series, only 3 (6%) of 48 IgG4-KD patients had renal lesions alone.
The clinical manifestations are usually mild or even asymptomatic. As the disease progresses, renal dysfunction or abnormalities are seen in imaging studies; only then it becomes clinically evident. Renal function at the time of the initial diagnosis varies from normal function to renal failure. Mild proteinuria occurs in approximately half the patients (710). Hypergammaglobulinemia, either IgG4 or total IgG, is the serologic hallmark of IgG4-KD as well as IgG4-SD. Although up to 30% of patients with IgG4-SD have a normal range of serum IgG4 level, almost all IgG4-KD patients have an elevated serum IgG4 level (710). Other serologic findings include an elevated serum immunoglobulin E level, eosinophilia, and hypocomplementemia (15).
Immunoglobulin G4-related kidney disease manifests most commonly as tubulointerstitial nephritis, followed by glomerular disease such as membranous glomerulonephritis (1). Other less frequent conditions, including IgG4-related chronic sclerosing pyelitis, IgG4-related plasma-cell arteritis, and IgG4-related inflammatory pseudotumors of the ureter, have also been reported (1141516). The key histologic findings of IgG4-KD are dense lymphoplasmacytic infiltration with increased IgG4-positive plasma cells, and storiform fibrosis, both of which are identical to the findings of IgG4-SD involving other organs (Fig. 1) (310). In addition, the characteristic microscopic features of IgG4-related tubulointerstitial nephritis are nests of inflammatory cells with irregular fibers surrounding them, and immune complex deposition in the tubular basement membrane (51718).
The diagnosis of IgG4-KD is based on a combination of imaging, serologic and histologic findings, as well as other organ involvement (57). Among these findings, the imaging findings may be the most important component as they are usually the first recognized abnormal findings of IgG4-KD, which enables clinicians or radiologists to consider the occurrence of this disease. Therefore, it is crucial to be aware of the imaging spectrum of IgG4-KD in order to obtain a timely and accurate diagnosis.
Immunoglobulin G4-related kidney disease can be divided into three types, based on their location: renal parenchymal, renal pelvic, and a perinephric lesion. A renal parenchymal lesion is the most common type, followed by a renal pelvic and a perinephric lesion (91219). Two types can sometimes appear together. Among the 48 IgG4-KD patients seen at our institution, 36 (75%) had only renal parenchymal lesions, 5 (10%) had only renal pelvic lesions, and 1 (2%) had only perinephric lesions. Two types of IgG4-KD co-existed in 6 (13%) patients: 5 with renal parenchymal and renal pelvic lesions, and 1 with renal parenchymal and perinephric lesions. Because of the systemic nature of the disease, IgG4-KD typically shows multiplicity and bilaterality (91920). In our series, 45 (94%) and 37 (77%) patients had multiple and bilateral lesions, respectively.
A renal parenchymal lesion in IgG4-KD can show three patterns: a single nodule, multiple nodules (Fig. 2), and diffuse patchy infiltrative lesions (Fig. 3). Among these, multiple nodules are the most common pattern (121920). Renal parenchymal nodules are located predominantly in the renal cortex. They are usually small and round, or wedge-shaped. It rarely appears as a single, large, mass-like lesion mimicking a solid renal neoplasm (91219).
On contrast-enhanced CT, renal parenchymal lesions are mostly hypodense to the normal renal cortex in the early phase, i.e., the arterial or corticomedullary phase, and progressively get enhanced in the portal or delayed phase (Fig. 2) (192021). These lesions are usually not visible on precontrast CT scans (1219). It can often be challenging to perceive early, subtle renal lesions depending solely on CT scans. MR imaging can improve detection of the lesions in such cases. On T2-weighted MR images, renal lesions typically appear hypointense relative to the normal renal parenchyma, and are easily detectable due to the obvious contrast between the dark lesions and the bright, normal parenchyma (Figs. 3, 4) (91222). On the other hand, renal lesions usually appear isointense on T1-weighted images, and they are barely visible or are invisible (Fig. 4). On dynamic contrast-enhanced MR images, most renal lesions are hypointense to the normal renal cortex in the arterial phase, and progressively enhance in the portal and delayed phases, thus becoming indistinct as the phase passes; this is similar to the dynamic enhancement pattern seen on CT (Fig. 5) (91222). Diffusion-weighted MR imaging (DWI) has recently been reported to be particularly useful for detecting IgG4-KD (9). Kim et al. (9) reported that the sensitivity of DWI (b value, 1000 s/mm2) for detecting IgG4-KD was significantly higher than that of T2-weighted images (100% vs. 77%). The renal lesions have marked hyperintensity on DWI at a high b value and, conversely, have marked hypointensity on the apparent diffusion coefficient map (Figs. 4, 5). These DWI findings can be explained by the histopathologic background of IgG4-KD, i.e., dense lymphoplasmacytic infiltration with fibrosis. This may be supported by the hypointensity seen on T2-weighted images and the progressive enhancement pattern seen on dynamic contrast-enhanced images, which may also represent fibrosis (912). DWI may also have the potential to detect IgG4-KD at an early or subclinical stage (923).
Ultrasonographic (US) findings of IgG4-KD are not specific and are less sensitive for lesion detection than CT or MR imaging (24). The most common US findings are ill-defined, non-mass-like areas of decreased echogenicity (24). Other US findings include irregular lobular thickening of the renal parenchyma with a bulging contour and multiple, hypoechoic masses with decreased vascularity, as compared to the normal renal cortex (24).
Renal pelvic or perinephric lesions are much rarer than renal parenchymal lesions. Renal pelvic lesions manifest as diffused wall thickening of the renal pelvis, or as a mildly enhanced, soft-tissue mass encasing the renal pelvis (1219). These lesions can be unilateral (Fig. 6) or bilateral (Fig. 7). Hydronephrosis seldom occurs, unless the ureter is entrapped by accompanying periaortic retroperitoneal fibrosis. In our series, only 1 of 10 patients with renal pelvic lesions had a mild, unilateral hydronephrosis without retroperitoneal fibrosis. Perinephric lesions typically appear as a diffuse, rim-like, soft-tissue mass surrounding the kidneys (Fig. 8) (12). These masses usually exhibit homogeneous and mild enhancement. They can be also unilateral or bilateral.
Immunoglobulin G4-related kidney disease usually shows a rapid and favorable response to steroid therapy, which similar to IgG4-SD with other organ involvement, regardless of the lesion location (i.e., renal parenchymal, renal pelvic or perinephric). According to the pathologic subtype, tubulointerstitial nephritis generally responds better than membranous glomerulonephritis (1). Approximately 90% of IgG4-KD patients show improvement of decreased renal function and abnormal imaging findings with steroid treatment (110121719). During the steroid treatment, the lesion size and number dramatically decreases, as seen on imaging studies (Fig. 9). After completion of the steroid therapy, varying degrees of renal atrophy can develop, especially in patients with renal parenchymal lesions, and which may be proportionate to the extent of the renal involvement before treatment (61012). In our series, the majority of patients having renal parenchymal lesions of a diffuse patchy infiltrative pattern, or with large, multiple nodules, experienced mild cortical scars after steroid therapy (Fig. 9). IgG4-KD as well as other organ involvement of IgG4-SD can relapse in up to 20% of patients, mainly during maintenance therapy or after cessation of the steroid therapy (Fig. 9) (612). Without steroid treatment, the renal lesions can increase in size and number, or progress to diffuse cortical involvement (12).
As stated above, IgG4-KD has a broad spectrum of imaging features. Therefore, various neoplastic or non-neoplastic conditions of the kidneys can mimic IgG4-KD. With regard to IgG4-KD manifesting as multiple, renal parenchymal lesions, the differential diagnosis includes acute pyelonephritis, infarction, metastasis, and lymphoma (Fig. 10) (91220). Acute pyelonephritis typically appear as unilateral or bilateral, multiple, ill-defined, and round or wedge-shaped lesions of decreased enhancement (Fig. 10A) (2526). Clinical findings, which include fever, pyuria or knocking tenderness in the flank area, may undoubtedly be important clues suggesting acute pyelonephritis. In addition to the aforementioned clinical findings, perilesional infiltration or fluid collection, and renal or perirenal abscesses seen on imaging examinations, may also help to differentiate acute pyelonephritis from IgG4-KD. Well-demarcated, large, wedge-shaped areas showing poor contrast enhancement are typical of renal infarction, although they may mimic the patchy infiltrative pattern of IgG4-KD (Fig. 10B) (2527). A hyperenhanced cortical rim at the lesion periphery, the so-called cortical rim sign, can be seen in approximately half of the patients with renal infarction, whereas in IgG4-KD this sign has never been reported in the published medical literature, including our study (2728). Metastases usually appear as bilateral, multiple, and less exophytic nodules, and they are therefore capable of closely mimicking IgG4-KD (Fig. 10C) (29). The presence of primary malignancy is the most important clue to metastasis. The common sites of primary malignancy are the lung, breast, contralateral kidney, and colon (30). Renal lymphomas commonly manifest as multiple, parenchymal nodules/masses (Fig. 10D), or as a renal pelvic or perinephric mass with mild homogeneous enhancement, which can be quite similar to the IgG4-KD features (313233). Moreover, lymphomas often appear hypointense on T2-weighted MR images and have diffusion restriction (3334). However, renal lymphomas seem more bulky than IgG4-KD and frequently accompany multiple, retroperitoneal lymphadenopathies, compared to IgG4-KD (932).
In rare cases, when IgG4-KD manifests as a single parenchymal nodule/mass, it can be challenging to distinguish it from renal cell carcinoma, especially when non-clear cell types, such as the papillary or chromophobe type, appear as a non-hypervascular mass (Fig. 11) (35). Diffuse wall thickening of the renal pelvis or a parapelvic mass in IgG4-KD should be differentiated from urothelial carcinoma (Fig. 12). The relatively common bilaterality and uncommon hydronephrosis in IgG4-KD may be helpful in differentiating the two diseases, and may warrant further comparative studies.
In addition to the above-mentioned analyses regarding the imaging features, detection of other organ involvement of IgG4-SD, i.e., AIP (Fig. 13), sclerosing cholangitis, retroperitoneal fibrosis (Fig. 13) or sialadenitis, may be essential for differentiating IgG4-KD from the other diseases, as isolated IgG4-KD without other organ involvement is very rare, as demonstrated in our study (6%). According to recently established diagnostic criteria of IgG4-KD, the presence of other organ involvement is an important diagnostic component (57). Alternatively, identification of renal lesions can be of crucial importance for the diagnosis of IgG4-SD involving other organs, especially when imaging features of other organs are not characteristic of IgG4-SD. A typical example would be the differential diagnosis of focal-type AIP and pancreatic cancer; in this situation, the presence of renal lesions may substantially favor the former diagnosis (Fig. 14) (8).
On the other hand, despite the detailed analysis using various imaging modalities, it can still be difficult to differentiate IgG4-KD from other diseases, in particular neoplasms. In such cases, CT- or US-guided biopsy of the renal lesions should be considered, in order to avoid unnecessary surgery caused by the misdiagnosis as neoplasm, in patients having IgG4-KD.
Immunoglobulin G4-related kidney disease has a broad spectrum of imaging features and a variety of mimickers to be differentiated from it. Awareness of the broad imaging spectrum of IgG4-KD, differential diagnosis from its mimickers, and important clinicopathologic characteristics, will facilitate its accurate and prompt diagnosis as well as optimally timed treatment.
Figures and Tables
Fig. 1
72-year-old man who underwent left nephrectomy due to IgG4-KD mimicking urothelial carcinoma.
A. Contrast-enhanced CT image shows ill-defined, homogeneous, soft-tissue mass (arrow) which encases left renal pelvis causing hydronephrosis. B. He underwent left nephrectomy with diagnosis of having urothelial carcinoma. Photograph of surgical specimen demonstrates lobulated, yellowish, firm mass (arrows) around renal pelvis. C, D. Lower-power (× 100) (C) and higher-power (× 400) (D) photomicrographs of histological specimen (hematoxylin and eosin staining) show dense plasma cell infiltration (arrows) with fibrous background (Fi) and obliterative phlebitis (arrowheads). E. IgG4 immunostaining (× 100) of histologic specimen shows numerous, brown, IgG4-positive plasma cells. IgG4-KD = immunoglobulin G4-related kidney disease
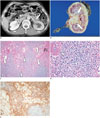
Fig. 2
49-year-old man with IgG4-KD manifesting as multiple, renal parenchymal nodules.
A, B. Contrast-enhanced arterial (A) and portal (B) phase CT images show multiple, small, round or wedge-shaped, hypodense nodules (arrows) in both kidneys, predominantly in cortex. Renal nodules progressively enhance in portal phase. IgG4-KD = immunoglobulin G4-related kidney disease
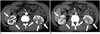
Fig. 3
IgG4-KD manifesting as diffuse patchy infiltrative parenchymal lesions.
A. 66-year-old man. Contrast-enhanced portal phase CT image shows patchy infiltrative hypodense lesions (arrows) in both kidneys. B. 56-year-old man. T2-weighted MR image shows diffuse patchy infiltrative lesions appearing noticeably hypointense (arrows) in both kidneys. IgG4-KD = immunoglobulin G4-related kidney disease
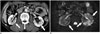
Fig. 4
MR imaging features of IgG4-KD in 64-year-old man.
A. On precontrast T1-weighted image, isointense renal lesions are not perceptible. B. T2-weighted image shows three, small, hypointense cortical nodules (arrows) in both kidneys. C, D. DWI (b value, 1000 s/mm2) (C) and ADC map (D) reveal renal nodules (arrows) which appear as having obvious hyperintensity and hypointensity, respectively, and representing diffusion restriction. ADC = apparent diffusion coefficient, DWI = diffusion-weighted MR imaging, IgG4-KD = immunoglobulin G4-related kidney disease
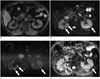
Fig. 5
MR imaging features of IgG4-KD in 72-year-old man.
A-C. Dynamic contrast-enhanced MR images show small cortical nodule (arrows) in right kidney, and which appears hypointense in arterial phase (A) and progressively enhanced in portal (B) and delayed (C) phases, gradually becoming indistinct as phase passes. D. On DWI (b value, 1000 s/mm2), right renal lesion exhibits striking hyperintensity (arrow). DWI = diffusion-weighted MR imaging, IgG4-KD = immunoglobulin G4-related kidney disease
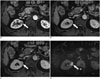
Fig. 6
64-year-old man with IgG4-KD manifesting as unilateral renal pelvic lesion.
Contrast-enhanced arterial phase CT image shows ill-defined, mildly enhancing, soft-tissue mass (arrow) encasing right renal pelvis and without hydronephrosis. IgG4-KD = immunoglobulin G4-related kidney disease
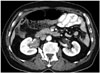
Fig. 7
84-year-old man with IgG4-KD manifesting as bilateral renal pelvic lesions.
Contrast-enhanced portal phase CT images show mildly enhanced wall thickening of both renal pelvises (arrows) and without hydronephrosis. Pancreatic head enlargement with heterogeneous attenuation (arrowheads), indicating autoimmune pancreatitis, is also seen. IgG4-KD = immunoglobulin G4-related kidney disease
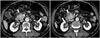
Fig. 8
74-year-old man with IgG4-KD manifesting as perinephric lesion.
Contrast-enhanced arterial phase CT image shows homogeneous, rim-like, soft-tissue mass surrounding both kidneys (arrows). Peri-aortic, soft-tissue lesion, indicating retroperitoneal fibrosis, is also observed (arrowhead). IgG4-KD = immunoglobulin G4-related kidney disease
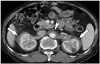
Fig. 9
Response of IgG4-KD to steroid therapy in 72-year-old man.
A. Contrast-enhanced CT image shows diffuse infiltrative lesion (arrows) in right kidney. B. Contrast-enhanced CT image obtained after completion of steroid treatment demonstrates improvement of right renal lesion. However, multifocal, tiny cortical scars remain (arrows). C. Contrast-enhanced CT image obtained two years after steroid cessation reveals nodular or infiltrative lesions in right kidney (arrows), thus indicative of disease relapse. IgG4-KD = immunoglobulin G4-related kidney disease
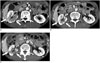
Fig. 10
Mimickers of IgG4-KD manifesting as multiple, renal parenchymal lesions.
A. 32-year-old woman with acute pyelonephritis. Contrast-enhanced delayed phase CT image shows multifocal, ill-defined, round and wedge-shaped, hypodense lesions (arrows) with mild swelling in left kidney. B. 53-year-old man with renal infarction. Contrast-enhanced arterial phase CT image shows large, rather well-demarcated, wedge-shaped areas of poor contrast enhancement (arrows) in both kidneys. C. 75-year-old man with renal metastases from lung cancer. Contrast-enhanced arterial phase CT image shows round, hypodense nodules (arrows) in both kidneys. D. 61-year-old man with renal lymphomas. Contrast-enhanced portal phase coronal CT image shows several, well-defined, homogeneous, round nodules/masses (arrows) in both kidneys. IgG4-KD = immunoglobulin G4-related kidney disease
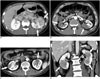
Fig. 11
Mimicker of IgG4-KD manifesting as single parenchymal nodule.
A, B. 78-year-old man with chromophobe-type renal cell carcinoma. Contrast-enhanced arterial (A) and delayed (B) phase CT images demonstrate single hypovascular nodule (arrows) in right kidney. IgG4-KD = immunoglobulin G4-related kidney disease
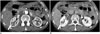
Fig. 12
Mimicker of IgG4-KD manifesting as renal pelvic lesion.
63-year-old man with transitional cell carcinoma. Contrast-enhanced portal phase CT image shows diffusely enhancing wall thickening of right renal pelvis (arrow) and surrounding soft-tissue lesion (arrowhead). IgG4-KD = immunoglobulin G4-related kidney disease
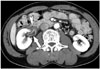
Fig. 13
Retroperitoneal fibrosis and autoimmune pancreatitis as clues to diagnosis of IgG4-KD in 71-year-old man.
A, B. Contrast-enhanced portal phase CT images show several, well-defined, round, hypodense nodules (arrows) in both kidneys. Differential diagnosis for these renal lesions would be lymphoma, metastasis or IgG4-KD. However, periaortic, soft-tissue lesion (arrowhead in A) suggesting retroperitoneal fibrosis and sausage-shaped pancreas swelling with peripancreatic hypodense rim (arrowheads in B) which is typical of AIP, are observed together. Accordingly, such renal lesions can confidently be diagnosed as IgG4-KD. AIP = autoimmune pancreatitis, IgG4-KD = immunoglobulin G4-related kidney disease
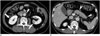
Fig. 14
IgG4-KD as clue for differentiating autoimmune pancreatitis from pancreatic cancer in 64-year-old man.
A, B. Precontrast T1-weighted MR image (A) shows hypointense, mass-like enlargement of pancreatic head (arrow). MR cholangiopancreatography (B) shows abrupt, severe narrowing of pancreatic and bile ducts (arrowheads) with upstream duct dilatation. These MR findings are highly suggestive of pancreatic cancer. C, D. DWIs (b value, 1000 s/mm2) show multifocal, hyperintense nodules (arrows) in right kidney. Combination of these renal lesions and pancreatic mass/enlargement strongly suggests IgG4-SD, i.e., IgG4-KD and focal type AIP. AIP = autoimmune pancreatitis, DWI = diffusion-weighted MR imaging, IgG4-KD = immunoglobulin G4-related kidney disease, IgG4-SD = IgG4-related sclerosing disease
References
1. Cornell LD. IgG4-related kidney disease. Semin Diagn Pathol. 2012; 29:245–250.
2. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012; 366:539–551.
3. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192.
4. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012; 22:21–30.
5. Raissian Y, Nasr SH, Larsen CP, Colvin RB, Smyrk TC, Takahashi N, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. 2011; 22:1343–1352.
6. Saeki T, Kawano M, Mizushima I, Yamamoto M, Wada Y, Nakashima H, et al. The clinical course of patients with IgG4-related kidney disease. Kidney Int. 2013; 84:826–833.
7. Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011; 15:615–626.
8. Khalili K, Doyle DJ, Chawla TP, Hanbidge AE. Renal cortical lesions in patients with autoimmune pancreatitis: a clue to differentiation from pancreatic malignancy. Eur J Radiol. 2008; 67:329–335.
9. Kim B, Kim JH, Byun JH, Kim HJ, Lee SS, Kim SY, et al. IgG4-related kidney disease: MRI findings with emphasis on the usefulness of diffusion-weighted imaging. Eur J Radiol. 2014; 83:1057–1062.
10. Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010; 78:1016–1023.
11. Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011; 23:57–66.
12. Takahashi N, Kawashima A, Fletcher JG, Chari ST. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007; 242:791–801.
13. Kim JH, Kim MH, Byun JH, Lee SS, Lee SJ, Park SH, et al. Diagnostic strategy for differentiating autoimmune pancreatitis from pancreatic cancer: is an endoscopic retrograde pancreatography essential. Pancreas. 2012; 41:639–647.
14. Kuroda N, Nakamura S, Miyazaki K, Inoue K, Ohara M, Mizuno K, et al. Chronic sclerosing pyelitis with an increased number of IgG4-positive plasma cells. Med Mol Morphol. 2009; 42:236–238.
15. Kim SA, Lee SR, Huh J, Shen SS, Ro JY. IgG4-associated inflammatory pseudotumor of ureter: clinicopathologic and immunohistochemical study of 3 cases. Hum Pathol. 2011; 42:1178–1184.
16. Sharma SG, Vlase HL, D'Agati VD. IgG4-related tubulointerstitial nephritis with plasma cell-rich renal arteritis. Am J Kidney Dis. 2013; 61:638–643.
17. Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int. 2014; 85:251–257.
18. Yamaguchi Y, Kanetsuna Y, Honda K, Yamanaka N, Kawano M, Nagata M. Japanese study group on IgG4-related nephropathy. Characteristic tubulointerstitial nephritis in IgG4-related disease. Hum Pathol. 2012; 43:536–549.
19. Triantopoulou C, Malachias G, Maniatis P, Anastopoulos J, Siafas I, Papailiou J. Renal lesions associated with autoimmune pancreatitis: CT findings. Acta Radiol. 2010; 51:702–707.
20. Hedgire SS, McDermott S, Borczuk D, Elmi A, Saini S, Harisinghani MG. The spectrum of IgG4-related disease in the abdomen and pelvis. AJR Am J Roentgenol. 2013; 201:14–22.
21. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011; 31:1379–1402.
22. Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011; 260:428–436.
23. Pozdzik AA, Matos C, Rorive S, Brocheriou I, Delhaye M, Nortier JL. Diffusion-weighted magnetic resonance imaging: a non-nephrotoxic prompt assessment of kidney involvement in IgG4-related disease. Kidney Int. 2014; 85:981.
24. Sasiwimonphan K, Gorman B, Kawashima A, Chari ST, Takahashi N. Renal involvement in patients with autoimmune pancreatitis: ultrasound findings. Eur J Radiol. 2012; 81:807–810.
25. Saunders HS, Dyer RB, Shifrin RY, Scharling ES, Bechtold RE, Zagoria RJ. The CT nephrogram: implications for evaluation of urinary tract disease. Radiographics. 1995; 15:1069–1085. discussion 1086-1088
26. Kawashima A, Sandler CM, Goldman SM, Raval BK, Fishman EK. CT of renal inflammatory disease. Radiographics. 1997; 17:851–866. discussion 867-868
27. Suzer O, Shirkhoda A, Jafri SZ, Madrazo BL, Bis KG, Mastromatteo JF. CT features of renal infarction. Eur J Radiol. 2002; 44:59–64.
28. Wong WS, Moss AA, Federle MP, Cochran ST, London SS. Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology. 1984; 150:201–205.
29. Honda H, Coffman CE, Berbaum KS, Barloon TJ, Masuda K. CT analysis of metastatic neoplasms of the kidney. Comparison with primary renal cell carcinoma. Acta Radiol. 1992; 33:39–44.
30. Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979; 72:806–807.
31. Ganeshan D, Iyer R, Devine C, Bhosale P, Paulson E. Imaging of primary and secondary renal lymphoma. AJR Am J Roentgenol. 2013; 201:W712–W719.
32. Jafri SZ, Bree RL, Amendola MA, Glazer GM, Schwab RE, Francis IR, et al. CT of renal and perirenal non-Hodgkin lymphoma. AJR Am J Roentgenol. 1982; 138:1101–1105.
33. Semelka RC, Kelekis NL, Burdeny DA, Mitchell DG, Brown JJ, Siegelman ES. Renal lymphoma: demonstration by MR imaging. AJR Am J Roentgenol. 1996; 166:823–827.
34. Saremi F, Knoll AN, Bendavid OJ, Schultze-Haakh H, Narula N, Sarlati F. Characterization of genitourinary lesions with diffusion-weighted imaging. Radiographics. 2009; 29:1295–1317.
35. Yamashita Y, Takahashi M, Watanabe O, Yoshimatsu S, Ueno S, Ishimaru S, et al. Small renal cell carcinoma: pathologic and radiologic correlation. Radiology. 1992; 184:493–498.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download