Abstract
Objective
The aims of this study were to compare opening and closing angles of normally functioning mechanical aortic valves measured on dual-source computed tomography (CT) with the manufacturers' values and to compare CT-measured opening angles according to valve function.
Materials and Methods
A total of 140 patients with 10 different types of mechanical aortic valves, who underwent dual-source cardiac CT, were included. Opening and closing angles were measured on CT images. Agreement between angles in normally functioning valves and the manufacturer values was assessed using the interclass coefficient and the Bland-Altman method. CT-measured opening angles were compared between normal functioning valves and suspected dysfunctioning valves.
Results
The CT-measured opening angles of normally functioning valves and manufacturers' values showed excellent agreement for seven valve types (intraclass coefficient [ICC], 0.977; 95% confidence interval [CI], 0.962-0.987). The mean differences in opening angles between the CT measurements and the manufacturers' values were 1.2° in seven types of valves, 11.0° in On-X valves, and 15.5° in ATS valves. The manufacturers' closing angles and those measured by CT showed excellent agreement for all valve types (ICC, 0.953; 95% CI, 0.920-0.972). Among valves with suspected dysfunction, those with limitation of motion (LOM) and an increased pressure gradient (PG) had smaller opening angles than those with LOM only (p < 0.05).
Prosthetic mechanical valve function is traditionally evaluated by transthoracic echocardiography (TTE) and cinefluoroscopy. However, a single TTE examination is limited because of suboptimal visualization of valve motion due to acoustic shadowing in the mechanical valve and wide variations among transprosthetic pressure gradients (PGs) (1). Although cinefluoroscopy enables a noninvasive evaluation of opening and closing angles of mechanical valve leaflets (2), obtaining a perpendicular image of the valve leaflet by fluoroscopy and accurately measuring the opening angle can be difficult (3).
Use of cardiac computed tomography (CT) is valuable for evaluating prosthetic heart valves, such as measuring opening and closing angles, diameter, and geometric orifice area (3456), and for diagnosing a prosthetic valve obstruction due to thrombosis, pannus, or vegetation (789). However, studies that have measured opening and closing angles by CT are limited based on a limited number of valve types among various commercially available mechanical valves. Moreover, cardiac CT has been reported useful to accurately measure opening and closing angles of mechanical valves compared to angle values reported by manufacturers (35) and angle values measured with cinefluoroscopy (346), but the normal range of CT-measured values is unavailable.
The aims of this study were to compare opening and closing angles measured on dual-source cardiac CT in normally functioning mechanical aortic valves with manufacturers' values, and to compare CT-measured opening angles according to valve function.
The Institutional Review Board of our institution approved this retrospective study, and informed consent was waived. We retrospectively searched a database of cardiac CT examinations performed from March 2010 to April 2014. Among 21440 consecutive patients who underwent CT during this period, 140 were included who had undergone aortic valve replacement (AVR) with a mechanical valve before CT scanning. Demographic data and information on the mechanical valves were collected from electronic medical records. Patients underwent cardiac CT for suspected coronary artery disease (n = 77), suspected prosthetic aortic valve dysfunction on TTE (n = 36), suspected dysfunction of the mitral or tricuspid valve on TTE (n = 11), postoperative evaluation after AVR (n = 7), pulmonary vein evaluation before radiofrequency ablation (n = 6), and evaluation of a coronary bypass graft (n = 3).
All CT scans were performed with a dual-source CT scanner (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany). In the absence of contraindications, patients with a heart rate > 65 beats/min received 50-mg of an oral beta-blocker (metoprolol tartrate) 1 hour before the examination and were administered a 0.3 mg sublingual dose of nitroglycerin just before the scan. Scans were performed using retrospectively electrocardiogram-gated data acquisition. The appropriate time interval between contrast agent injection and scanning initiation was determined for each patient by the timing bolus technique. After a bolus injection of 10 mL of iopamidol (Pamiray: 370 mg iodine/mL; Dongkook Pharma, Seoul, Korea) followed by 20 mL saline at 5 mL/s, optimal delay times were determined by automatically evaluating contrast enhancement in the ascending aorta. All CT scans were performed using a triple-phase injection method (70 mL iopamidol followed by 30 mL 30% blended iopamidol with saline and 20 mL saline at 5 mL/s). Tube potential and tube current-time product from March 2010 to July 2011 were designated according to a body mass index-based protocol described in previous studies (1011). From Automatic tube potential selection with tube current modulation was used with the CARE kV™ software (Siemens Healthcare) and simultaneous application of CARE Dose4D (Siemens Healthcare) beginning in August 2011 (1011).
Images were reconstructed with a medium kernel (b36f), and reconstruction slice thickness was 0.75 mm with 0.5-mm increments. Ten transverse datasets were reconstructed for every 10% of the cardiac cycle in all patients. The reconstructed images were transferred to an image server and analyzed using dedicated three-dimensional software (Aquarius iNtuition, Ver 4.4.11, TeraRecon, San Mateo, CA, USA).
All CT analyses were performed by two radiologists, who were blinded to clinical information. In cases of disagreement, a final assessment was reached through consensus. The mechanical valves were evaluated using multiplanar reformatted images in cine mode. The default vertebrae window was selected to visualize the mechanical valves, with additional adjustment to the window level and width to minimize blooming at the discretion of the reader. Three common CT views were created for analysis. A short-axis image of the mechanical aortic valve was created in a direction similar to that of the surgeon's view. A long-axis view of the left ventricular outflow tract was created in parallel to the axis of the mechanical valve leaflets, and the coronal-section view was created perpendicular to the left ventricular long-axis view.
The CT assessment of a mechanical aortic valve consisted of evaluating leaflet motion and pannus formation. The opening and closing angles (defined as the angle between the leaflet and the orifice ring) were measured in the fully open and closed positions to evaluate leaflet motion (Fig. 1) (3). Limitation of motion (LOM) was defined when one of the following two criteria was met: 1) motion of a leaflet or leaflets was consistently restricted, or 2) the opening angle measured on CT decreased > 4° compared to the manufacturer value, as described previously (12). The ATS pivot and On-X valves were the exceptions. An opening angle of the ATS pivot valve that decreased > 20° (< 65°) from the manufacturer value (85°) was considered a decrease, as reported in a previous study using cinefluoroscopy (13). LOM of the On-X valve was assessed based on the consistently restricted motion of a leaflet or leaflets without using a cutoff because a decrease in the opening angle > 9° (< 81°) from the manufacturer's value (90°) was reported in an in vitro study using cinefluoroscopy (14). The presence of a subprosthetic soft tissue mass (pannus) was assessed. If a pannus was present, pannus severity was assessed and classified as insignificant or significant. A pannus was significant if the diameter of the subprosthetic portion narrowed by the pannus was > 50% of the internal diameter of the geometric orifice area of the mechanical valve as assessed visually.
We classified the valves into groups of normally functioning and suspected dysfunction for the data analysis. We defined normally functioning valves as those that did not show a LOM, and that had a normal range of mean transprosthetic PG (≤ 30 mm Hg) on TTE (15). Valves with suspected dysfunction met one of the following conditions: 1) LOM on CT or 2) elevated PG (> 30 mm Hg) on TTE (16). When LOM could not be evaluated on the CT scan because of a severe artifact, PG measured on TTE was used for the classification. We subclassified valves with suspected dysfunction into three groups (LOM only, PG elevation only, and both LOM and PG elevation). The LOM only group was defined as valves showing LOM on CT and a normal PG range (≤ 30 mm Hg) on TTE. The PG elevation only group was defined when the valve did not show LOM on CT but had an elevated PG. The both LOM and PG elevation group was defined as valves showing LOM and elevated PG. Valves with elevated PG and in-assessable image quality for LOM were classified into the PG elevation only group.
Statistical analyses were performed using the MedCalc for Windows ver. 12.7.0.0 program (MedCalc Software, Mariakerke, Belgium). Normally distributed data were identified using the Shapiro-Wilk W test. Continuous variables are presented as mean ± standard deviation and were compared using the independent t test for normally distributed data or the Mann-Whitney U-test for non-normally distributed data. Statistical significance of categorical variables was investigated with chi-square statistics. Ordinal variables are presented as medians with 25-75% interquartile ranges (IQRs) and were compared using the Kruskal-Wallis analysis of variance. Interobserver variability for measuring opening and closing angles was assessed using the intraclass coefficient (ICC). The paired t test or Wilcoxon signed-rank test was used to compare the manufacturers' values of normally functioning valves with those determined by CT. The ICC was used to assess the agreement between the opening and closing angles measured on CT and the manufacturers' values. The limits of agreement according to Bland and Altman were determined to compare opening and closing angles between the manufacturers' values and CT measurements. Pearson's correlation coefficient analysis was used to detect correlations between differences in CT-measured opening angles and manufacturers' values and transvalvular PGs on TTE. The Mann-Whitney U-test was used to compare opening and closing angles and TTE parameters between normally functioning valves and valves with suspected dysfunction. A p value < 0.05 was considered significant.
The final study population consisted of 140 patients, and their clinical and CT information is summarized in Table 1. Ten different mechanical valve types were included (135 bileaflet and five tilting disc valves; 28 Carbomedics, 26 St. Jude Medical Regent, 23 St. Jude Medical, 15 ATS open pivot, 12 On-X, 11 Duromedics, 11 Sorin, nine MIRA, three Björk-Shiley, and two Medtronic Hall).
Measuring the opening and closing angles of the mechanical valves was not feasible in some patients because of poor image quality during the systolic or diastolic phase, and the presence of LOM could not be evaluated in 16 cases. The opening angles of three Björk-Shiley (100%), five Duromedics (45.5%), one Carbomedics (3.6%), and one ATS (6.7%) valve could not be measured because of severe beam-hardening artifacts. The closing angles of three Björk-Shiley (100%), six Duromedics (54.5%), one St. Jude Medical Regent (3.8%), one St. Jude Medical (4.3%), one ATS (6.7%), and one On-X (8.3%) valve could not be measured. The opening and closing angles measured on CT were medians of 78.7° (IQR, 73.4-82.8°) and 25.4° (IQR, 23.1-29.6°), respectively. The ICCs between two observers for measuring the opening and closing angles were 0.990 (95% confidence interval [CI], 0.986-0.993) and 0.979 (95% CI, 0.970-0.985), respectively. The mean differences between the two observers for measuring the opening and closing angles were 0.2° and 0°, respectively. A total of 93 valves were considered normally functioning based on the CT and TTE results, and the remaining 47 valves were had suspected dysfunction (six showed LOM only, 23 had an elevated PG, and 18 showed both LOM and an elevated PG) (Table 2).
The opening angles of normally functioning valves measured on CT scans were significantly different from the manufacturers' values for St. Jude Medical Regent, ATS, On-X, and MIRA valves (p < 0.05) (Table 3). However, the difference in St. Jude Medical Regent, and MIRA valves was too small to be clinically significant. The ICC between the manufacturers' values and the opening angles measured on CT was 0.321 (95% CI, 0.118-0.498). After excluding the ATS and On-X valves, the ICC was 0.951 (95% CI, 0.920-0.970), representing excellent agreement. The mean differences between the opening angles on CT and the manufacturers' values were 1.0° in seven types of valves, excluding the Björk-Shiley, ATS, and On-X valves, 11.0° in the On-X valves, and 15.5° degrees in the ATS valves (Fig. 2).
The closing angles measured on CT scans were not significantly different from the manufacturers' values for most of the valve types, except the St. Jude Medical Regent, Carbomedics, Sorin, and MIRA valves. The ICC between the closing angles reported by the manufacturer and those on CT measurements was 0.956 (95% CI, 0.933-0.972). The mean difference between the closing angles on CT and the manufacturers' values was 0.2°.
The difference between opening angles measured on CT and the manufacturer value was correlated with peak and mean PG on TTE (r = 0.143, p = 0.107 for peak PG; r = 0.176, p < 0.05 for mean PG) (Fig. 3), particularly after excluding the Björk-Shiley, ATS, and On-X valves (r = 0.409 for peak PG and 0.453 for mean PG; p < 0.001).
The median differences between the opening angles measured on CT and the manufacturers' values were 1.52°, 5.03°, 1.22°, and 12.4° for normally functioning valves in the three groups (LOM only, PG elevation only, and both LOM and PG elevation groups), respectively (Figs. 4, 5). The presence of a pannus and significant pannus were significantly different between the four groups (p < 0.001, respectively). Median transvalvular PGs were 15.0, 15.5, 41.0, and 41.5 mm Hg, respectively. Among valves with suspected dysfunction, the both LOM and PG elevation group had significantly smaller opening angles than those of the LOM only group (p = 0.036). The PG elevation only group had a lower incidence of significant pannus (52.4%, 11/21) than that of the LOM only group (80.0%, 4/5) and both the LOM and PG elevation group (94.4%, 17/18), respectively. The PG elevation group and the both LOM and PG elevation group showed no significant difference in mean PG.
Our results demonstrate that dual-source cardiac CT provides accurate measurements for opening and closing angles in most types of mechanical aortic valves, compared with values reported by their manufacturers.
Previous studies have reported the utility of cardiac CT for measuring opening and closing angles in limited types of mechanical valves, such as the St. Jude Medical, Carbomedics, and Medtronic Hall valves (345). However, no data are available regarding opening and closing angles in other types of valves or the normal ranges of values measured on CT. In our study, most normally functioning valves showed similar opening and closing angles as those reported by the manufacturers (± 1°). ATS and On-X valves were the exceptions with significantly smaller opening angles than those reported by their manufacturers. Although the closing angles of the St. Jude Medical Regent, Carbomedics, Sorin, and MIRA valves measured on CT were significantly different from the manufacturers' values, the differences were too small to be clinically significant.
The reason why the ATS and On-X valves showed larger differences between the opening angles on CT and the manufacturers' values than other valve types could be explained by the structural features of these two valve types. Cinefluoroscopic studies have demonstrated that the leaflet opening angles of normally functioning ATS valves in vivo are less than those observed in vitro and reported by the manufacturer (13). Two mechanisms have been suggested to explain this in vivo leaflet movement of the ATS valve: an effect caused by a local flow field induced by the unique open pivot and/or an effect caused by decreased opening time when the outlet of the ATS valve is abruptly enlarged to the orifice ring diameter (1417). On-X valves have been known to show a not-fully-open phenomenon in vitro without hemodynamic compromise because of the expanding configuration of the outlet to the orifice ring, a phenomenon similar to that of the ATS valves (14).
In our study, the opening and closing angles of all Björk-Shiley valves and some Duromedics valves could not be measured due to severe beam-hardening artifacts from the valve leaflets. These results were consistent with those of previous studies (418), reporting that valves containing a cobalt-chrome alloy such as the Björk-Shiley, Duromedics, and Sorin tilting disc valves, produce severe beam-hardening artifacts that prevent an assessment of mechanical valves. However, current mechanical valves are made of titanium and carbon rather than a cobalt-chrome alloy. Therefore, the limited utility of CT for evaluating these cobalt-chrome alloy valve types may not be a serious limitation in the future. Other than these two valve types, some cases were encountered in which CT measurements could not be made due to severe beam-hardening artifacts, particularly in patients with arrhythmias during the CT scan. Although our results suggest that valve type is an important factor affecting CT image quality, further study is needed to determine the actual factors affecting CT image quality when assessing mechanical valves.
Cinefluoroscopy is the modality of choice to assess leaflet motion of mechanical aortic valves because it is superior to echocardiography for identifying leaflet motion (19). However, cinefluoroscopy requires proper patient positioning with the X-ray beam parallel to both the valve ring and the tilting axis of the leaflets; performing this examination is time-consuming and obtaining images with the patient in the correct position is frequently difficult (20). If the valve is not properly oriented, valve opening can be over or underestimated on cinefluoroscopy. In contrast, the valve can be reoriented in any CT plane. CT could offer optimal visualization of valve profiles independent of valve position, the patient's physical characteristics, limitation of C-arm motion, and the operator skill, compared with fluoroscopy.
The positive correlation we observed between transvalvular PG and the differences between CT-measured opening angles and the manufacturers' values was consistent with a previous cinefluoroscopic study (21), suggesting that a decrease in opening angle may suggest elevated PG on TTE. However, this result was not applied to the PG elevation only group among valves with suspected dysfunction. Interestingly, the both LOM and PG elevation group had a significantly decreased opening angle compared to that of the LOM only group, as well as more significant formation of pannus compared with that of the PG elevation only group. Differences in opening angles in the different valve function subgroups can be explained by the hemodynamic effect of pannus formation, which depends on the extent and site of fibrous tissue (2223). A pannus arising from the neointima in the periannulus of the left ventricular septum (24) may extend to the orifice and hinges of a prosthetic valve and result in restricted leaflet motion or may cause severe stenosis due to obstruction of the left ventricular outflow tract by a circumferential pannus without restricting leaflet motion (2223). Additionally, LOM due to a circumferential pannus can be explained by the effect caused by decreased opening time due to abrupt enlargement of the outlet to the narrowed left ventricular outflow tract by a pannus, similar to the not-fully-open phenomenon of ATS and On-X valves.
In addition to evaluating opening and closing of valve leaflets, CT can be used to measure annulus diameter and geometric orifice area and provide further information about a mechanical valve, such as the cause for dysfunction, by thrombus, pannus, or vegetation (7912). CT can complement TTE and cinefluoroscopy during a functional and morphological evaluation of a mechanical aortic valve by combining the measured opening and closing angle values and other information, such as valve size and the presence of a thrombus or pannus.
Our study had several limitations. First, measuring angles on CT may have resulted in errors, if the reconstruction plane was not oriented correctly. However, we analyzed interobserver variability for the measurements and obtained clinically acceptable interobserver agreement. Second, the CT data were limited to 10 axial datasets with 10% step increments of the RR interval across the cardiac cycle. This raises the possibility that the maximal opening point on systole may have been missed in some patients. Radiation exposure is another limitation of examining mechanical heart valves with CT. In particular, radiation dose may have been an issue in our study because most CT scans were performed with retrospective gating without modulating tube current, regardless of the indications for the CT scan.
In conclusion, dual-source CT was an accurate modality to assess opening and closing angles of most types of mechanical aortic valves. Opening and closing angles measured on CT were about 1° different compared to manufacturer-reported values, except those for ATS and On-X valves. Measurements of the Björk-Shiley and Duromedics valves were hampered by severe beam-hardening artifacts. Opening angles measured on CT differed according to the type of valve dysfunction, and a decrease in opening angle may suggest an elevated PG on TTE, except in cases showing only a PG elevation without LOM due to formation of a significant pannus.
Figures and Tables
Fig. 1
Examples of dual-source CT images for measuring opening and closing angles in normally functioning mechanical aortic valves.
CT images show measurement of opening and closing angles (A-C) of normal functioning mechanical aortic valves. C. Measurements of DM (opening angle) and BS valves (opening and closing angles) could not be performed due to severe artifacts. BS = Björk-Shiley, CT = computed tomography, DM = Duromedics, MH = Medtronic-Hall, SJM = St. Jude Medical, SJR = St. Jude Medical Regent
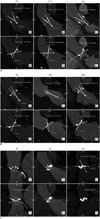
Fig. 2
Bland-Altman plots to compare CT opening angle measurements and those of manufacturer.
Mean differences between opening angles on CT and manufacturers' values were 4.2° for all valve types (except Björk-Shiley valves) (A) and 1.0° for seven valve types other than Björk-Shiley, ATS, and On-X valves (B). CT = computed tomography

Fig. 3
Correlation between transvalvular PG and opening angles.
Scatter plots show positive correlations between mean PG and opening angles in all valve types, except Björk-Shiley valves (n = 128, r = 0.176, p = 0.047) (A) and seven valve types, except Björk-Shiley, ATS, and On-X valves (n = 103, r = 0.453, p < 0.001) (B). PG = pressure gradient

Fig. 4
CT measurements and TTE parameters between valve function groups.
Box and whisker plots of (A) differences between manufacturers' values and opening angles on CT for all valve types and (B) in seven valves, except Björk-Shiley, ATS and On-X valves show significant differences between groups with different valve function*. Box and whisker plot of (C) mean PG according to valve function shows significant difference in PG between groups†. Presence of pannus (D) and significant pannus (E) are significantly different between four groups on CT. *Except between normally functioning valves and PG elevation only group, †Except between normally functioning valves and LOM only group and between PG elevation group and both LOM and PG elevation group. CT = computed tomography, LOM = limitation of motion, PG = pressure gradient, TTE = transthoracic echocardiography

Fig. 5
CT measurements in Carbomedics valves based on valve function.
Long axis view CT images (top and middle) show opening and closing angles of Carbomedics valves in aortic position, and short axis view images (bottom) show subprosthetic area in valves with normal function and suspected dysfunction (LOM only, PG elevation only, and both LOM and PG elevation). Valve with suspected dysfunction and both LOM and PG elevation shows greater decrease in opening angle than that of valve with LOM only. LOM = limitation of motion, PG = pressure gradient
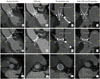
Table 1
Clinical Characteristics and CT Parameters

Table 2
Classification of Study Population According to Valve Types and Results of CT and TTE

Table 3
CT Measurement for Opening and Closing Angles in Normally Functioning Valves According to Valve Type

References
1. Bach DS. Echo/Doppler evaluation of hemodynamics after aortic valve replacement: principles of interrogation and evaluation of high gradients. JACC Cardiovasc Imaging. 2010; 3:296–304.
2. Carrier M, Pellerin M, Basmadjian A, Bouchard D, Perrault LP, Cartier R, et al. Fifteen years of clinical and echocardiographic follow up with the carbomedics heart valve. J Heart Valve Dis. 2006; 15:67–72. discussion 72
3. LaBounty TM, Agarwal PP, Chughtai A, Bach DS, Wizauer E, Kazerooni EA. Evaluation of mechanical heart valve size and function with ECG-gated 64-MDCT. AJR Am J Roentgenol. 2009; 193:W389–W396.
4. Konen E, Goitein O, Feinberg MS, Eshet Y, Raanani E, Rimon U, et al. The role of ECG-gated MDCT in the evaluation of aortic and mitral mechanical valves: initial experience. AJR Am J Roentgenol. 2008; 191:26–31.
5. Lee DH, Youn HJ, Shim SB, Lee SH, Jung JI, Jung SE, et al. The measurement of opening angle and orifice area of a bileaflet mechanical valve using multidetector computed tomography. Korean Circ J. 2009; 39:157–162.
6. Suchá D, Symersky P, Vonken EJ, Provoost E, Chamuleau SA, Budde RP. Multidetector-row computed tomography allows accurate measurement of mechanical prosthetic heart valve leaflet closing angles compared with fluoroscopy. J Comput Assist Tomogr. 2014; 38:451–456.
7. Tsai IC, Lin YK, Chang Y, Fu YC, Wang CC, Hsieh SR, et al. Correctness of multi-detector-row computed tomography for diagnosing mechanical prosthetic heart valve disorders using operative findings as a gold standard. Eur Radiol. 2009; 19:857–867.
8. Teshima H, Hayashida N, Fukunaga S, Tayama E, Kawara T, Aoyagi S, et al. Usefulness of a multidetector-row computed tomography scanner for detecting pannus formation. Ann Thorac Surg. 2004; 77:523–526.
9. Ueda T, Teshima H, Fukunaga S, Aoyagi S, Tanaka H. Evaluation of prosthetic valve obstruction on electrocardiographically gated multidetector-row computed tomography--identification of subprosthetic pannus in the aortic position. Circ J. 2013; 77:418–423.
10. Park YJ, Kim YJ, Lee JW, Kim HY, Hong YJ, Lee HJ, et al. Automatic Tube Potential Selection with Tube Current Modulation (APSCM) in coronary CT angiography: Comparison of image quality and radiation dose with conventional body mass index-based protocol. J Cardiovasc Comput Tomogr. 2012; 6:184–190.
11. Suh YJ, Kim YJ, Hong SR, Hong YJ, Lee HJ, Hur J, et al. Combined use of automatic tube potential selection with tube current modulation and iterative reconstruction technique in coronary CT angiography. Radiology. 2013; 269:722–729.
12. Symersky P, Budde RP, de Mol BA, Prokop M. Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol. 2009; 104:1128–1134.
13. Aoyagi S, Arinaga K, Fukunaga S, Tayama E, Kosuga T, Akashi H. Leaflet movement of the ATS valve in the aortic position: unique behavior observed in 19-mm valves. Ann Thorac Surg. 2006; 82:853–857.
14. Feng Z, Nakamura T, Fujimoto T, Umezu M. In vitro investigation of opening behavior and hydrodynamics of bileaflet valves in the mitral position. Artif Organs. 2002; 26:32–39.
15. Parnell A, Swanevelder J. High transvalvular pressure gradients on intraoperative transesophageal echocardiography after aortic valve replacement: what does it mean? HSR Proc Intensive Care Cardiovasc Anesth. 2009; 1:7–18.
16. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009; 22:975–1014. quiz 1082-1084
17. Tayama E, Feng Z, Oda T, Tomoeda H, Hayashida N, Fukunaga S, et al. ATS prosthetic valve motion: an in vitro analysis. J Heart Valve Dis. 2000; 9:408–414.
18. Habets J, Mali WP, Budde RP. Multidetector CT angiography in evaluation of prosthetic heart valve dysfunction. Radiographics. 2012; 32:1893–1905.
19. Habets J, Budde RP, Symersky P, van den Brink RB, de Mol BA, Mali WP, et al. Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol. 2011; 8:466–478.
20. Montorsi P, Arena V, Muratori M, Lavarra F, Cavoretto D, Repossini A, et al. Fluoroscopic functional evaluation of bileaflet prostheses: effect of different intraoperative valve orientation. Am J Card Imaging. 1996; 10:101–107.
21. Cianciulli TE, Lax JA, Beck MA, Cerruti FE, Gigena GE, Saccheri MC, et al. Cinefluoroscopic assessment of mechanical disc prostheses: its value as a complementary method to echocardiography. J Heart Valve Dis. 2005; 14:664–673.
22. Cianciulli TF, Saccheri MC, Lax JA, Guidoin R, Zhang Z, Guerra JE, et al. Intermittent acute aortic regurgitation of a mechanical bileaflet aortic valve prosthesis: diagnosis and clinical implications. Eur J Echocardiogr. 2009; 10:446–449.
23. Kuniyoshi Y, Koja K, Miyagi K, Shimoji M, Uezu T, Arakaki K, et al. Pannus formation in aortic valve prostheses in the late postoperative period. J Artif Organs. 2003; 6:179–182.
24. Teshima H, Hayashida N, Yano H, Nishimi M, Tayama E, Fukunaga S, et al. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg. 2003; 126:401–407.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download