Abstract
Materials and Methods
This study included five pathologically proven cases of IAC, and their sonographic features were retrospectively analyzed according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon.
Results
All five lesions involved the left breast and were seen as irregularly shaped masses. All lesions, except one, had a parallel orientation to the chest wall. All five lesions showed noncircumscribed margins and heterogeneous echotexture; however, they showed various posterior features. One lesion had edema as an associated feature. Sonographic assessments were classified as BI-RADS category 4 in all five cases.
Invasive apocrine carcinoma (IAC) is a distinctive and rare type of breast cancer that was first described by Krompecher in 1916 (1). Microscopically, IAC demonstrates the same architectural growth pattern as invasive ductal carcinoma (IDC) of no special type, differing only in its apocrine cytological features. Using Rosen's strict definition, no more than 5% of breast carcinomas are classified as IAC (2). The clinical presentation is similar to that of IDC of no special type (3). A few radiology reports have been published regarding this disease entity, although, to the best of our knowledge, there are only two published case reports regarding its sonographic characteristics (45). Therefore, the objectives of this study were to present and describe the sonographic results of five detected IAC cases of the breast with at least 90% apocrine differentiation on hematoxylin and eosin (H&E) staining. A relevant review of the medical literature is included as well.
Appropriate Institutional Review Board approval was obtained from all involved institutions, and patient informed consent was waived. Patient records from the pathology registry of the radiology departments at four academic hospitals were reviewed. Originally, the total number of patients with imaging-studied IAC from 2010 to 2013 was 14. However, when we classified those cases according to our criteria, i.e., a carcinoma with at least 90% apocrine-cell differentiation seen after H&E staining, five of the 14 patients were ultimately included in our study.
All of the patients were postmenopausal women ranging in age from 48 to 73 years. In all of the study patients, imaging studies had been requested for a palpable lump in the breast. There were no symptoms such as mastalgia or discharge. All patients underwent breast-conserving surgery with sentinel and axillary lymph-node dissection. The range of apocrine cells in carcinoma was 90% to 100%. There was no evidence of metastasis in the lymph nodes of any patient. Immunohistochemistry testing was also performed and revealed no expression of estrogen receptor (ER) or progesterone receptor (PR) proteins. All five cases showed positive results for gross cystic disease fluid protein 15 (GCDFP-15) (Table 1).
Both mammography and sonography were performed in all patients, and only one patient underwent breast MRI and positron emission tomography-CT (PET-CT). In all five patients, the lesions were discovered on the initial diagnostic mammography. Sonography was performed after mammography for further evaluation in all patients.
The sonographic findings were retrospectively analyzed. Lesion size, shape, orientation, margin, echotexture, posterior features, and associated features were evaluated according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon (6).
The sonographic findings are summarized in Table 1. Lesions ranged in longitudinal size from 1.2 to 2.2 cm. All five lesions involved the left breast, and were seen as irregularly shaped masses. All lesions, except one, had a parallel orientation to the chest wall. All five lesions had noncircumscribed margins and heterogeneous echotexture; however, they all showed various posterior features. One lesion had edema as an associated feature. Sonographic assessments were classified as BI-RADS category 4 in all five cases (Figs. 1, 2, 3).
Microscopically, IAC demonstrates the same architectural growth pattern as IDC of no special type, differing only in its cytological appearance. The cells are characterized by typical apocrine features: abundant, eosinophilic granular cytoplasms and prominent, and often multiple, nucleoli.
According to reports published before 1980, the incidence of IAC varies from < 0.3% to 4% (7). However, as there was a lack of standardized diagnostic criteria for IAC before 1980 and there still seems to be no established diagnostic criteria accepted by all pathologists (8), it would be correct to assume that the incidence of IAC is rare, even though the exact incidence is still unknown.
The reported patient age range of IAC is quite wide (19-86 years), although some reports have suggested a higher incidence in postmenopausal women. There are no appreciable differences between apocrine and non-apocrine tumors with regard to their bilaterality, location within the breast, stage at diagnosis, or familial history (3).
When we searched the medical literature for IAC sonographic findings, we could only find two case reports due to the rarity of this tumor. In a case report by Onoue et al. (4), where the IAC appeared as two cysts, they appeared as hypoechoic cysts and a hyperechoic papillary projection was noted in the larger cyst. In another case report by Gokalp et al. (5), two different IAC foci showed distinct characteristics on sonography, one as a solid lesion with a spiculated angular margin and the other in the form of a complex cyst containing solid components or thick septa. In our patients, no lesion appeared as a cyst on sonography, and all lesions were seen as irregularly shaped, solid masses with heterogeneous internal echo patterns and noncircumscribed margins.
There was only one published study regarding IAC mammography. Through a mammography review of 16 cases of IAC and one case of apocrine ductal carcinoma in situ, Gilles et al. (9) concluded that the mammographic pattern of IAC does not differ from those of common IDC. Of these 17 cases, 10 had microcalcifications associated with tumors.
We were able to find only limited information regarding the MRI of IAC in three articles (101112). Based on these cases, we could not find any differences between IAC and more common carcinomas of ductal origin.
Image findings of our five IACs are in line with previous results. Our cases have findings that are difficult to differentiate from those of IDC of no special type. However, we have presented new imaging sets of IAC in five cases, as previously published sonography cases have findings substantially different from those of our patients, and there is limited mammography and MRI data. To the best of our knowledge, aside from our study, no PET-CT feature of IAC has been reported so far.
Interestingly, apocrine tumors showing prominent apocrine changes demonstrate a unique immunohistochemical profile, frequently with a strongly expressed androgen receptor (AR), although seldom with expressed ER/PR (13). Although little is known about the role of AR and its prognostic value, the presence of a high AR positivity rate (56-100%) in these apocrine tumors could perhaps expand future therapeutic options for these women. GCDFP-15 is a major component of aspirated cyst fluid and is characteristic of apocrine cells, although its expression is not always associated with an apocrine morphology, i.e., GCDFP-15 is expressed in 75% of apocrine tumors and in 23% of non-apocrine breast carcinomas (14). In apocrine tumors, the rate of human epidermal growth factor receptor 2 (HER2) overexpression is between 44-54% and P53 has been reported in approximately 50% of apocrine tumors.
Honma et al. (15) reported that ER/PR/HER2 negativity can be helpful to confirm the diagnosis of IAC because it is more consistent than GCDFP-15/AR positivity.
Currently, not only immunohistochemistry but also molecular profiling is widely used in breast cancer classification. Tsutsumi (16) advocated for immunohistochemical categorization, finding it superior to the conventional histological criteria for diagnosing IAC. They concluded immunohistochemically-defined apocrine-type carcinoma as ER (-)/PR (-)/AR (+) may show clinical behaviors different from the triple-negative breast cancer.
There seems to be no difference in treatment options. Regarding the response to chemotherapy, a published study in which differences in the histological type of breast cancer and response to neoadjuvant chemotherapy were studied, IAC responded poorly (17). A case of triple-negative IAC showed a complete pathologic response to neoadjuvant chemotherapy (18). However, the number of cases was not sufficient to reach any definitive conclusions.
In an article by Japaze et al. (19), the severity of axillary lymph node involvement was statistically significant between the IAC and IDC patient groups (a median of three lymph nodes vs. five, respectively).
The prognosis of IAC is not yet clear. Tanaka et al. (20) and d'Amore et al. (21) indicated that IAC showed no significant difference in the overall patient survival rate compared with IDC; however, Japaze et al. (19) suggested that patient survival at six years after diagnosis was significantly better in IAC and that it may be a distinct clinicopathological entity with less aggressive behavior than IDC and therefore might be regarded as an independent prognostic factor in early breast cancer.
We have presented the sonography results of five IAC cases with at least 90% apocrine differentiation on H&E staining. All our cases have sonographic findings that make them difficult to be differentiated from that of IDC of no special type. They also demonstrated a consistent immunohistochemistry of ER (-)/PR (-)/GCDFP-15 (+).
Figures and Tables
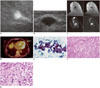 | Fig. 1Invasive apocrine carcinoma of breast in 73-year-old woman (case 1).
A. Mediolateral oblique mammography showing irregularly shaped, hyperdense mass with partially indistinct margin. B. Sonography scan shows irregularly shaped, heterogeneous echotexture mass with microlobulated margin and combined posterior shadowing (BI-RADS category 4). C. MRI obtained before lumpectomy shows irregular shape and margin mass. This mass was seen as having homogeneous, low-signal intensity on T1- (T1WI) and T2-weighted images (T2WI) and showed rapid, heterogeneous enhancement during initial phase and wash-out except for anterior rim in delayed phase on dynamic contrast study (T1WI, T2WI, early, and delayed enhancement phases; clockwise direction). D. Entire tumor showed moderate FDG uptake (SUV = 3.2) on PET-CT. E. Lesion aspiration showed few irregular syncytial fragments composed of pleomorphic, malignant apocrine cells. Cells showed abundant granular cytoplasm and large, eccentric nuclei with irregular chromatin distribution. Note multiple prominent macronuclei (Papanicolaou, × 100). F. Microscopically, tumor showed irregular tumor nests infiltrating stroma. Note abundant eosinophilic cytoplasm and apocrine feature of tumor cells with definite nuclear pleomorphism, which is pathognomonic of apocrine carcinoma (H&E stain, × 100). G. Most tumor cells demonstrated prominent apocrine features as well as abundant eosinophilic, granular cytoplasm and marked nuclear pleomorphism (H&E stain, × 400). BI-RADS = Breast Imaging Reporting and Data System, FDG = fluorodeoxyglucose, H&E = hematoxylin and eosin, PET-CT = positron emission tomography-CT, SUV = standardized uptake value
|
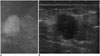 | Fig. 2Invasive apocrine carcinoma of breast in 65-year-old woman (case 3).
A. Mediolateral oblique mammography showing irregularly shaped, hyperdense mass with partially indistinct margin. B. Sonography showing irregular shape and heterogeneous echotexture mass with indistinct margin. Posterior enhancement with peritumoral edema is noted (BI-RADS category 4). BI-RADS = Breast Imaging Reporting and Data System
|
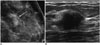 | Fig. 3Invasive apocrine carcinoma of breast in 48-year-old woman (case 5).
A. Craniocaudal mammography showing asymmetry (arrow). B. Sonography showing irregularly shaped, heterogeneous echotexture mass with indistinct margin (BI-RADS category 4). BI-RADS = Breast Imaging Reporting and Data System
|
Table 1
Summary of Pathology and Sonography Findings in Five Invasive Apocrine Carcinomas of Breast

References
1. Krompecher E. Zur Histogenese und Morphologie der Cystenmamma (maladie kystique reclus, cystadenoma Schimmelbuscin, mastitis chronica cystica Konig) des intrakanalikularen Kystadenomas und der Kystadenokarzinome der Brustdruse (Hidrokystoma, kystadenoma, Hidrokystadenocarzinoma mammae). Beitr Pathol Anat. 1916; 62:403–410.
2. Eusebi V, Millis RR, Cattani MG, Bussolati G, Azzopardi JG. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol. 1986; 123:532–541.
3. Bhargava R. Apocrine carcinoma of the breast. In : Dabbs DJ, editor. Breast pathology. Philadelphia, PA: Elsevier/Saunders;2012. p. 502–511.
4. Onoue S, Katoh T, Chigira H, Matsuo K, Suzuki M, Shibata Y, et al. A Case of Apocrine Carcinoma of the Breast Presenting as Two Cysts. Breast Cancer. 1997; 4:193–196.
5. Gokalp G, Topal U, Haholu A, Kizilkaya E. Apocrine carcinoma of the breast: mammography and ultrasound findings. Eur J Radiol Extra. 2006; 60:55–59.
6. Mendelson EB, Böhm-Vélez M, Berg WA. ACR BI-RADS Ultrasound. American College of Radiology. ACR BI-RADS breast imaging and reporting data system. 5th ed. Reston, VA: American College of Radiology;2013.
7. Azzopardi JG, Ahmed A, Millis RR. Problems in breast pathology. Philadelphia: Saunders;1979. p. 57–67. p. 341–344.
8. Vranic S, Schmitt F, Sapino A, Costa JL, Reddy S, Castro M, et al. Apocrine carcinoma of the breast: a comprehensive review. Histol Histopathol. 2013; 28:1393–1409.
9. Gilles R, Lesnik A, Guinebretière JM, Tardivon A, Masselot J, Contesso G, et al. Apocrine carcinoma: clinical and mammographic features. Radiology. 1994; 190:495–497.
10. Yuen S, Uematsu T, Kasami M, Tanaka K, Kimura K, Sanuki J, et al. Breast carcinomas with strong high-signal intensity on T2-weighted MR images: pathological characteristics and differential diagnosis. J Magn Reson Imaging. 2007; 25:502–510.
11. Kuroki-Suzuki S, Kuroki Y, Nasu K, Nawano S, Moriyama N, Okazaki M. Detecting breast cancer with non-contrast MR imaging: combining diffusion-weighted and STIR imaging. Magn Reson Med Sci. 2007; 6:21–27.
12. Hirose M, Hashizume T, Seino N, Kubota H, Nobusawa H, Gokan T. Atlas of breast magnetic resonance imaging. Curr Probl Diagn Radiol. 2007; 36:51–65.
13. Tavassoli FA, Purcell CA, Bratthauer GL, Man YG. Androgen receptor expression along with loss of bcl-2, ER, and PR expression in benign and malignant apocrine lesions of the breast: implications for therapy. Breast J. 1996; 2:261–269.
14. Mazoujian G, Bodian C, Haagensen DE Jr, Haagensen CD. Expression of GCDFP-15 in breast carcinomas. Relationship to pathologic and clinical factors. Cancer. 1989; 63:2156–2161.
15. Honma N, Takubo K, Akiyama F, Sawabe M, Arai T, Younes M, et al. Expression of GCDFP-15 and AR decreases in larger or node-positive apocrine carcinomas of the breast. Histopathology. 2005; 47:195–201.
16. Tsutsumi Y. Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012; 42:375–386.
17. Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 2012; 21:289–295.
18. Iizuka M, Enomoto K, Sakurai K. [A case of breast cancer treated with neoadjuvant chemotherapy and segmentectomy]. Gan To Kagaku Ryoho. 2012; 39:2027–2029.
19. Japaze H, Emina J, Diaz C, Schwam RJ, Gercovich N, Demonty G, et al. 'Pure' invasive apocrine carcinoma of the breast: a new clinicopathological entity? Breast. 2005; 14:3–10.
20. Tanaka K, Imoto S, Wada N, Sakemura N, Hasebe K. Invasive apocrine carcinoma of the breast: clinicopathologic features of 57 patients. Breast J. 2008; 14:164–168.
21. d'Amore ES, Terrier-Lacombe MJ, Travagli JP, Friedman S, Contesso G. Invasive apocrine carcinoma of the breast: a long term follow-up study of 34 cases. Breast Cancer Res Treat. 1988; 12:37–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download