Abstract
Objective
To evaluate the safety and efficacy of unilateral covered stent placement in patients with malignant superior vena cava (SVC) syndrome.
Materials and Methods
Between October 2008 and November 2012, expanded polytetrafluoroethylene-covered stent placement for malignant SVC syndrome was performed in 40 consecutive patients (35 men and five women; mean age, 61.4 years; range, 35-81 years). All covered stents were unilaterally placed within the SVC or across the venous confluence when needed to relieve venous obstruction and prevent tumor overgrowth, regardless of patency of contralateral brachiocephalic veins.
Results
Stent placement was technically successful in all patients. There were no major complications. Of the 37 patients symptomatic prior to stent placement, 34 (92%) experienced complete symptomatic relief 1-8 days after stent placement. Of the 29 patients who underwent covered stent placement across the venous confluence, nine patients had patent contralateral brachiocephalic veins prior to stent placement. However, no sign of SVC obstruction or contralateral upper extremity venous thrombosis was observed during the follow-up period. Kaplan-Meier analysis revealed median patient survival of 163 days. Stent occlusion occurred in four (10%) of 40 patents. Cumulative stent patency rates at 1, 3, 6, and 12 months were 95%, 92%, 86%, and 86%, respectively.
Superior vena cava (SVC) syndrome is a common complication of malignancy, with lung cancer being the predominant cause (1). It is evident in 4% of lung cancer patients at diagnosis, and it may also develop during the disease course (2). Patients with malignant SVC syndrome are typically seriously ill, and further deterioration is likely due to the presence of unresectable advanced malignant tumors. Although bypass surgery has been reported for palliative treatment of malignant SVC syndrome in selected patients, this type of surgery in terminally ill patients is difficult to justify and is rather invasive for a palliative procedure (3). Although radiotherapy and chemotherapy have been traditionally considered standards in the management of malignant SVC syndrome, reports of their effectiveness are controversial and both treatments have a slow response rate (4, 5).
Endovascular stents are currently used as the firstline therapeutic measure in patients with malignant SVC syndrome, because stenting does not interfere with subsequent antitumor treatment and provides urgent relief of symptoms (6). Prior studies using various types of uncovered stents reported the efficacy of stent placement in patients with malignant SVC syndrome (7-12).
A few reports have described the use of covered stents in recurrent SVC syndrome after uncovered stent placement or iatrogenic injury of the SVC (7-10). However, covered stents should be used with caution because of risks of stent migration and occlusion of contralateral brachiocephalic vein when the stent has to be placed across the confluence of the brachiocephalic vein.
The purpose of the present study was to evaluate the safety and efficacy of unilateral covered stent placement in patients with malignant SVC syndrome.
From October 2008 to November 2012, retrospectively collected data of 40 consecutive patients who underwent expanded polytetrafluoroethylene (ePTFE)-covered stent placement at the interventional unit for malignant SVC syndrome were evaluated. Demographic, clinical, and laboratory data were collected from the medical records of all 40 patients. This retrospective study was approved by the Institutional Review Board of our institution and written consent from each patient was waived.
The study group included 35 men and five women (mean age, 61.4; range, 35-81 years), all of whom had histologically confirmed malignant etiology (Table 1). A pathologic diagnosis of the underlying malignancy was made by biopsy, since treatment approaches could vary widely depending on the histology of the malignancy. Pathologic specimens were obtained by either bronchoscopy (n = 25) or percutaneous needle biopsy (n = 15). The majority of SVC obstructions were caused by lung cancer: small cell lung cancer in 10 patients and non-small cell lung cancer in 26 patients. Of the remaining four patients, thymic neoplasm was the underlying cause in three and metastatic mediastinal lymphadenopathy from neuroendocrine carcinoma in the remaining patient.
The diagnosis of malignant SVC syndrome was based on clinical signs and symptoms and imaging studies, such as contrast enhanced computed tomography (CT), of the chest and upper extremity venography. Aggravated swelling of the face and neck was evident in 37 patients with/without arm edema (n = 19), dyspnea (n = 18), or voice hoarseness (n = 2). Three patients who had not yet developed overt symptoms despite total SVC obstruction discovered on CT were also included in the study. In all 40 patients, stenosis greater than 80% or occlusion of SVC was identified by CT and venography prior to stent placement. While the obstructed venous segment was localized within the SVC in 15 patients, SVC as well as confluence of bilateral brachiocephalic veins was involved in the remaining 25 patients. Of those 25 patients with obstruction of the confluence, 20 patients showed obstruction of both brachiocephalic veins, whereas the remaining five patients showed unilateral obstruction of the branchiocephalic vein.
Malignant SVC syndrome was the first symptom of cancer in eight patients, whereas the diagnosis of cancer had already been ascertained in the other 32 patients. Of these 40 patients, 24 had received chemotherapy, nine had received chemotherapy and radiotherapy, and one had received radiotherapy alone. Patients with malignant SVC syndrome who did not receive prior chemotherapy and radiotherapy, or who failed either therapy were included in the study group. Of the 40 patients, 30 received primary stent placement prior to chemotherapy or radiotherapy for initial palliation of SVC syndrome, whereas the remaining 10 received secondary stent placement following the failure of primary oncologic therapies. Patients with malignant SVC syndrome and extensive thrombosis of the internal jugular or upper extremity venous system with uncorrectable coagulopathy, or with poor general health status (Eastern Cooperative Oncology Group performance status grade 3 or 4) were excluded.
Contrast venography and stent insertion were performed by two experienced interventional radiologists who each had more than 10 years of experience. Interventional procedures were performed under moderate sedation using intravenous pethidine hydrochloride (Demerol, Keukdong Pharmaceuticals, Seoul, Korea) and local anesthesia using intramuscular 2% lidocaine (Jeil Pharmaceuticals, Daegu, Korea) with continuous cardiopulmonary monitoring. Prophylactic antibiotics were not administered before or after the procedure.
A single right femoral venous access was used whenever possible, and internal jugular access was used as an alternative route when technically required. After puncture of the selected venous route, a 9 Fr or 10 Fr sheath (Cook, Bloomington, IN, USA) was inserted. A 0.035-inch angled hydrophilic guidewire (Terumo, Tokyo, Japan) and a 5 Fr catheter (Cobra or Headhunter; Cook) was then passed through the stenosis. Contrast venography was performed to evaluate the location, length, and severity of the stenosis or occlusion, followed by pressure gradient measurement between the venous segments proximal and distal to the lesion. Afterwards, hydrophilic guidewire was exchanged for 0.035-inch, 145-cm, or 260-cm extra stiff Amplatz guidewire (Cook). Pre-stenting dilatation using a 6-12-mm-diameter balloon catheter (Synergy; Boston Scientific, Galway, Ireland) was performed to determine the stent size and to facilitate easy navigation of the lesion as well as placement of the stent.
The triple-layered ePTFE-covered stent (ComVi stent; TaeWoong Medical, Gimpo, Korea) (11) used in this study was composed of ePTFE membrane between two uncovered nitinol self-expanding metallic stents. The wire was exposed on both the inner and outer surfaces of the stent. The stent was a partially ePTFE-covered stent with 0.5-cm at the proximal and distal bare extensions to prevent stent migration. The stent was available in diameters of 10-, 12-, or 14-mm and lengths of 6-10 cm. The 10- and 12-mm-diameter covered stents were accompanied by an 8.5 F introducing system, while the 14-mm-diameter covered stent was accompanied by a 9-F introducing system. The stent shortened by 15% after deployment.
As for prevention of the stent migration, selected stent diameter was always 10-15% larger than the SVC diameter, estimated using of both preprocedural enhanced CT scan and intraprocedular venogram. To prevent tumor overgrowth, the stent was placed so that it extended over both sides of the lesion by at least 1 cm. If the obstruction concerned only the SVC with sufficient tumor-free landing zone (more than 1 cm from the venous confluence), a covered stent was positioned within the SVC. When the proximal margin of the involved SVC was too close to the venous confluence (within 1 cm from the venous confluence) or when the obstruction directly involved the venous confluence without obstructing the brachiocephalic veins, the stent was unilaterally deployed so that it covered a right brachiocephalic vein and the SVC. This principle was consistently applied to patients who showed patent flow of the contralateral left brachiocephalic vein despite obstruction of the venous confluence. If thrombus or obliteration in the right or left brachiocephalic vein were detected upon contrast venography during the procedure and/or CT performed before stent placement, a unilateral stent was placed in the SVC and patent contralateral brachiocephalic vein.
Contrast venography was performed immediately after stent placement to confirm the appropriate positioning of the stent and pressure gradient was measured to determine the immediate effectiveness of stenting. If stent dilatation was insufficient or the pressure gradient was > 10 mm Hg, post-stenting balloon dilatation using an 8-12 mm-diameter balloon catheter was performed. Use of anticoagulation after the stent deployment was decided case-by-case by the clinicians, considering patients underlying medical conditions. Of the 40 patients, all but 13 received oral anticoagulation after stent placement.
Study endpoints were rates of technical success, complications, clinical success, as well as patient survival times, and stent patency. Technical success was defined as successful placement of the stent in an adequate position across the SVC obstruction and pressure gradient between the stent < 10 mm Hg. Complications were classified as major and minor according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee (12). Clinical success was defined as a major improvement or complete alleviation of symptoms. In patients with dyspnea, persistent dyspnea alone was not equated with clinical failure because it may be a symptom of the underlying pulmonary disease and is frequently found in patients with tumor invasion into the bronchus or pulmonary vessels.
Patient survival was defined as the time interval between stent placement and death or last follow-up. Stent patency was assumed if the cardinal symptoms of SVC syndrome did not occur and occlusion of the stent was not observed by contrast enhanced CT of the chest during the follow-up period. Of the total study population, all but eight patients underwent at least one enhanced chest CT exam. Follow-up venography was not routinely performed unless the patient required additional intervention. At the time of death, the stent was assumed to be patent if the patient had not developed the cardinal symptoms of SVC syndrome. If the patient had obvious symptoms of SVC syndrome, the stent was assumed to be obstructed. If there was no evidence of stent obstruction during the patient's life, the stent patency period was considered to be equal to the survival period.
Paired-sample t test was used to compare the pre- and post-stenting pressure gradients. Patient survival and stent patency rates were calculated using the Kaplan-Meier method. A p value < 0.05 indicated statistical significance. All statistical analyses were performed with SPSS version 11.0 for Windows (SPSS Inc., Chicago, IL, USA).
Stent placement was technically successful in all 40 patients (Fig. 1). Contrast venography immediately after stent placement confirmed the correct positioning of the stent and none of the stents migrated immediately after deployment. Post-stenting balloon dilatation was performed in all patients. The pressure gradient, which was 18.7 ± 5.7 mm Hg before stent placement, dropped significantly to 5.5 ± 3.0 mm Hg after stent placement (p < 0.001). Therefore, technical success rate was 100%. In seven patients, the stent was placed covering the SVC and left brachiocephalic vein, due to direct tumor invasion or total thrombosis of right brachiocephalic vein and internal jugular vein. Six patients (15%) experienced minor complications, including mild fever in five patients and hoarseness of voice in one patient. Five patients with mild fever showed favorable clinical courses within 2 days after antibiotic treatment. One patient complained of transient voice hoarseness after the stent placement, but was returned to normal 3 days after the procedure without any treatment. No major complications were observed.
In 25 patients with occlusion involving the confluence of brachiocephalic veins, a unilateral covered stent was placed in the SVC and brachiocephalic vein. In addition, four of 15 patients with obstruction localized within the SVC also required covered stent placement across the confluence because proximal margin of the involved SVC was too close to the confluence (less than 1 cm). Therefore, 29 patients underwent covered stent placement across the venous confluence. In 20 of the 29 patients, unilateral covered stent placement across the venous confluence was of little significance because they already presented with thrombus or significant narrowing of bilateral brachiocephalic veins prior to the stent placement. However, remaining nine patients had patent flow of the contralateral brachiocephalic vein prior to stent placement. In these nine patients, follow-up CT after unilateral covered stent placement revealed newly apparent obstruction of the contralateral brachiocephalic vein, either by thrombosis or obliteration. Obstruction was limited to the contralateral brachiocephalic vein in eight of nine patients, while only one patient showed obstruction extending to level of the scanned proximal axillary and cephalic veins. However, none of these patients exhibited apparent signs or symptoms of SVC obstruction or upper extremity venous thrombosis until the end of the study or patient death.
Of 37 patients who were symptomatic prior to stent placement, 34 experienced symptomatic improvement 1-8 days (mean, 2.3 days) after stent placement, but three patients did not experience any evident symptomatic relief after stent placement. Two of these patients did not experience symptomatic improvement due to early death within 5 days after stent placement. Another patient persistently complained of mild facial edema after a technically successful covered stent placement. Therefore, clinical success was achieved in 34 (92%) of 37 patients.
All patients were clinically followed-up until death or the end of the study. The cut-off date for data analysis was November 31, 2012. With a mean follow-up period of 175 days (range, 3-873 days), 31 (77.5%) patients had died. Seven (17.5%) patients died within 30 days after stent placement. According to Kaplan-Meier life-table analysis, median patient survival was 163 days (95% confidence interval 137-189 days) (Fig. 2).
Stent occlusion occurred in four (10%) of 40 patients after a mean of 45 days (range, 5-171 days). All stent occlusions were detected using enhanced chest CT scans and in two cases additional venography was performed, which confirmed stent occlusions. The occlusion was caused by intrastent thrombosis (n = 2) and tumor overgrowth (n = 2). Of the four patients, three were treated with balloon dilatation and subsequent additional covered stent placement and experienced symptomatic relief for 39, 141, and 244 days until death occurred. The other patient did not undergo intervention because of poor general condition. One case of stent occlusion occurred 5 days after stent placement in a patient who underwent anticoagulation therapy due to intrastent thrombosis. According to Kaplan-Meier life-table analysis, cumulative stent patency rates at 1, 3, 6, and 12 months were 95%, 92%, 86%, and 86%, respectively (Fig. 3).
Although endovascular stents have been traditionally offered to salvage recurrent malignant SVC obstruction after failure of other primary therapies, such as chemotherapy and radiation therapy, the approach is now considered firstline therapy in an increasing number of institutions (13-16). Stent placement provides relief from the venous obstruction in an immediate and direction fashion, resulting in rapid symptom relief within 24-72 hours following the procedure (16, 17). Endovascular stent insertion of malignant SVC syndrome is reported to improve symptoms in 81-100% of cases, which is superior to chemotherapy and radiotherapy (16, 18, 19). Furthermore, deployment of a stent as the first step has little or no influence on the decision of the oncologist to continue with scheduled therapies including chemotherapy and radiation therapy (16).
Although many studies have reported the efficacy of endovascular stenting in patients with malignant SVC syndrome, recurrence following initial successful stenting still occurs in up to 41% of cases, and is attributed to tumor ingrowth within the stent or venous thrombosis (2, 16, 19-21). However, most patients with malignant SVC syndrome have a short life expectancy and the stent remains patent until death. Furthermore, stent occlusion can be treated with thrombolysis, balloon dilatation, or further stent insertion with good secondary patency rates (2, 22). However, secondary endovascular treatments are sometimes difficult and have additional costs. Thus, to prevent stent occlusion and improve the patency rate of stents, we speculated that it may be important to prevent tumor ingrowth into the stents.
The vast majority of prior reports regarding endovascular stenting for SVC syndrome used uncovered metallic stents, rather than covered stents, to relieve SVC obstruction (15, 16, 23). There have been only five separate case reports of endovascular covered stent insertion in the SVC (7-11). Gill et al. (9) reported that placement of covered stent was able to maintain long term patency of SVC, after two bare stent insertions that resulted in reocclusion due to tumor ingrowth.
In our study, all covered stents were successfully deployed in an adequate position and all pressure gradients between the stent were < 10 mm Hg. Covered stent provided fast and effective relief of the SVC obstruction, demonstrating 100% technical success rate and 92% clinical success rate. Although stent migration is a significant complication, none of the patients experienced stent migration. This could be due to partially covered stent design with both bare extensions and existence of outer bare stent. Moreover, to avoid stent migration, the diameter of the covered stent was chosen to be 10-15% greater than the SVC diameter.
One important concern of covered stent placement was that when it is placed over the venous confluence the brachiocephalic veins, contralateral brachiocephalic vein occlusion could potentially lead to thrombosis of the upper extremity veins. A total of 29 patients underwent covered stent placement across the venous confluence. However, none displayed signs of SVC obstruction or contralateral upper extremity venous thrombosis during follow-up. None of 20 patients who already had bilateral brachiocephalic vein obstruction displayed signs of SVC obstruction after unilateral covered stent placement. Therefore, unilateral brachiocephalic vein revascularization was sufficient in these patients. Some investigators have reported that unilateral stent placement is preferable in patients with malignant SVC syndrome because it is as clinically effective as bilateral placement while offering lower cost, easier placement, and low rates of complications and recurrence (16, 17, 24, 25). In our study, unilateral covered stent placement yielded satisfactory results and inserting stents bilaterally was not necessary. Even though covered stent placement resulted in iatrogenic occlusion of contralateral brachiocephalic vein in nine patients who had patent flow of the contralateral brachiocephalic vein, none of these nine patients exhibited signs of SVC obstruction or signs of upper extremity venous thrombosis until the end of the study or patient death. This suggests that unilateral relief of obstruction may allow sufficient collateral flow.
Stent occlusion occurred in four (10%) of 40 patients after a mean of 55 days (range, 5-171 days) and cumulative stent patency rates at 1, 3, 6, and 12 months were 95%, 92%, 86%, and 86%, respectively. Except for one patient whose general condition rapidly deteriorated, regardless of the cause of stent occlusion, three patients were successfully treated with additional covered stent placement. Use of anticoagulant drugs showed no relationship with stent occlusion. In fact, stent thrombosis also occurred in a patient who was prescribed anticoagulation therapy after stent placement. Although many authors believe that anticoagulation therapy is mandatory after stent placement, its effectiveness has never been clearly proven (14, 16, 23, 26). The question of the appropriate antithrombotic preventive strategy remains to be resolved.
We found that technical success rate (100%), clinical success rate (92%), and stent occlusion rate (10%) of unilateral uncovered stent placement were in agreement with the results of previous studies (13-17, 19, 22, 24, 27). Also, Gwon et al. (28) recently reported in a single-center comparative cohort study that unilateral placement of covered stents were associated with higher cumulative stent patency rates and lower stent occlusion rates than uncovered stents for treating SVC syndrome. Although their study population included only three patients who had unilateral brachiocephalic vein and SVC obstruction, their result further supports the use of uncovered stent in the clinical setting of malignant SVC syndrome.
There are several limitations of this study. This was a retrospective study and there are innate limitations of such study designs. Secondly, there were no control groups. Even though there is currently no consensus on the most effect interventional method to treat malignant SVC syndrome, comparative analysis between other procedures such as uncovered stent placement would be beneficial. Further randomized studies of other methods for treating SVC syndrome in larger numbers of patients are required. Thirdly, follow-up venogram was not available for all patients to confirm the stent occlusion. However, enhanced chest CT scans were available for 32 of 40 patients and patients were closely monitored by clinicians for any symptoms and signs of SVC syndrome. Nevertheless, because the stent was considered patent unless symptoms recurred, there is a possibility for overestimation of the stent patency. However, performing routine CT scans or venograms in asymptomatic patients would have been problematic due to excessive radiation, increased medical cost, and invasiveness of additive diagnostic procedures. Lastly, potential risk of overestimating the safety of the procedure must be mentioned. Despite through retrospective gathering of information, because there was no preset systematic protocol to assess the adverse events, some adverse events might have been unnoticed. Also, determining the exact cause of death based on death certificate is sometimes insufficient without postmortem autopsies.
In conclusion, unilateral covered stent placement appears to be a safe and effective method for treating malignant SVC syndrome, despite the location of SVC occlusion. Therefore, we believe that unilateral covered stent placement can be considered the first treatment option for patients with malignant SVC syndrome.
Figures and Tables
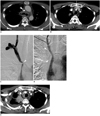
Fig. 1
52-year-old man with adenocarcinoma of lung that caused symptomatic SVC syndrome.
A. Contrast-enhanced axial CT image shows bronchus-encasing mass and mediastinal lymphadenopathy, causing significant narrowing of right SVC (arrowhead). B. Axial CT scan at upper level of (A) shows patent flow of left brachiocephalic vein demonstrated by contrast enhancement (arrows). C. Right internal jugular venography shows severe stenosis at right SVC (arrowhead) with proximal venous contrast stasis. D. Venography obtained after ePTFE-covered stent placement (12 mm × 4 cm) (arrowheads) across SVC and venous confluence shows fluent passage of contrast medium via stent. E. Follow-up contrast-enhanced axial CT image at same level of (B) performed 2 months after stent placement shows obliterated left brachiocephalic vein (arrows). However, patient was free of any symptoms related to SVC or left upper extremity venous obstruction. SVC = superior vena cava, ePTFE = expanded poly tetrafluoroethylene
References
1. Reechaipichitkul W, Thongpaen S. Etiology and outcome of superior vena cava (SVC) obstruction in adults. Southeast Asian J Trop Med Public Health. 2004; 35:453–457.
2. Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol). 2002; 14:338–351.
3. Picquet J, Blin V, Dussaussoy C, Jousset Y, Papon X, Enon B. Surgical reconstruction of the superior vena cava system: indications and results. Surgery. 2009; 145:93–99.
4. Dyet JF, Nicholson AA, Cook AM. The use of the Wallstent endovascular prosthesis in the treatment of malignant obstruction of the superior vena cava. Clin Radiol. 1993; 48:381–385.
5. Urban T, Lebeau B, Chastang C, Leclerc P, Botto MJ, Sauvaget J. Superior vena cava syndrome in small-cell lung cancer. Arch Intern Med. 1993; 153:384–387.
6. Smayra T, Otal P, Chabbert V, Chemla P, Romero M, Joffre F, et al. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol. 2001; 24:388–394.
7. Azizzadeh A, Pham MT, Estrera AL, Coogan SM, Safi HJ. Endovascular repair of an iatrogenic superior vena caval injury: a case report. J Vasc Surg. 2007; 46:569–571.
8. Chin DH, Petersen BD, Timmermans H, Rösch J. Stent-graft in the management of superior vena cava syndrome. Cardiovasc Intervent Radiol. 1996; 19:302–304.
9. Gill K, Ettles DF, Nicholson AA. Recurrent superior vena caval obstruction due to invasion by malignant thymoma: treatment using a stent-graft. Br J Radiol. 2000; 73:1015–1017.
10. Mansour M, Altenburg A, Haage P. Successful emergency stent implantation for superior vena cava perforation during malignant stenosis venoplasty. Cardiovasc Intervent Radiol. 2009; 32:1312–1316.
11. Gwon DI, Paik SH. Successful treatment of malignant superior vena cava syndrome using a stent-graft. Korean J Radiol. 2012; 13:227–231.
12. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003; 14:S199–S202.
13. Bierdrager E, Lampmann LE, Lohle PN, Schoemaker CM, Schijen JH, Palmen FM, et al. Endovascular stenting in neoplastic superior vena cava syndrome prior to chemotherapy or radiotherapy. Neth J Med. 2005; 63:20–23.
14. Gross CM, Krämer J, Waigand J, Uhlich F, Schröder G, Thalhammer C, et al. Stent implantation in patients with superior vena cava syndrome. AJR Am J Roentgenol. 1997; 169:429–432.
15. Nagata T, Makutani S, Uchida H, Kichikawa K, Maeda M, Yoshioka T, et al. Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Intervent Radiol. 2007; 30:959–967.
16. Lanciego C, Pangua C, Chacón JI, Velasco J, Boy RC, Viana A, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009; 193:549–558.
17. Nicholson AA, Ettles DF, Arnold A, Greenstone M, Dyet JF. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997; 8:781–788.
18. Watkinson AF, Yeow TN, Fraser C. Endovascular stenting to treat obstruction of the superior vena cava. BMJ. 2008; 336:1434–1437.
19. Nguyen NP, Borok TL, Welsh J, Vinh-Hung V. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax. 2009; 64:174–178.
20. Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006; 29:319–322.
21. Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi R. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol. 2009; 71:343–349.
22. Kee ST, Kinoshita L, Razavi MK, Nyman UR, Semba CP, Dake MD. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998; 206:187–193.
23. Fagedet D, Thony F, Timsit JF, Rodiere M, Monnin-Bares V, Ferretti GR, et al. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Intervent Radiol. 2013; 36:140–149.
24. Dinkel HP, Mettke B, Schmid F, Baumgartner I, Triller J, Do DD. Endovascular treatment of malignant superior vena cava syndrome: is bilateral wallstent placement superior to unilateral placement? J Endovasc Ther. 2003; 10:788–797.
25. Urruticoechea A, Mesía R, Domínguez J, Falo C, Escalante E, Montes A, et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion. Experience on 52 patients with lung cancer. Lung Cancer. 2004; 43:209–214.
26. Wan JF, Bezjak A. Superior vena cava syndrome. Hematol Oncol Clin North Am. 2010; 24:501–513.
27. Furui S, Sawada S, Kuramoto K, Inoue Y, Irie T, Makita K, et al. Gianturco stent placement in malignant caval obstruction: analysis of factors for predicting the outcome. Radiology. 1995; 195:147–152.
28. Gwon DI, Ko GY, Kim JH, Shin JH, Yoon HK, Sung KB. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology. 2013; 266:979–987.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download