Abstract
Objective
The aim of our study was to evaluate the differences between sclerotherapy with and without ethanol concentration monitoring for the treatment of simple renal cysts.
Materials and Methods
Sixty-seven patients with 70 simple renal cysts were randomly assigned to two groups in a 12-month prospective controlled trial. One group (group A) was treated with computed tomography (CT)-guided sclerotherapy without ethanol concentration monitoring (33 patients with 35 cysts), whereas the other group (group B) had ethanol concentration monitoring (34 patients with 35 cysts) during the procedure. Treatment outcomes between the two groups were compared 12 months later with follow-up ultrasound examination.
Results
After the 12-month follow-up period, the overall success rate was 74.3% in group A and 94.3% in group B (p = 0.022). The mean cyst size before and after treatment was 8.6 ± 2.0 cm and 2.3 ± 2.9 cm, respectively, in group A, and 8.4 ± 1.7 cm and 0.8 ± 1.9 cm, respectively, in group B. The final size of the cysts in group B was significantly smaller than that in group A (p = 0.015). The likelihood of treatment with ethanol concentration monitoring being successful was approximately 16 times higher than without ethanol concentration monitoring (p = 0.026; odds ratio = 15.7; 95% confidence interval: 1.38-179.49). There were no major complications in either group.
Simple renal cysts are the most common renal masses, and occur in half of all patients older than 50 years. Simple renal cysts are rarely symptomatic and necessitate no treatment (1). Treatment may be required if cyst growth leads to symptoms such as flank pain, hypertension, hematuria, infection or compression of the pelvicaliceal system. Percutaneous aspiration and sclerotherapy have been described as safe and effective methods of managing symptomatic simple renal cysts without the cost and morbidity associated with conventional surgery and laparoscopy (2-4).
Simple aspiration is associated with a high recurrence rate because the cyst wall epithelium is responsible for active liquid production (1). Percutaneous aspiration with single or multiple session sclerotherapy has successfully been performed with high success rates. Several sclerosing agents have been used to exterminate the cyst wall epithelium, including ethanol (1-3, 5, 6), polidocanol (4), acetic acid (7), OK-432 (8), ethanolamine oleate (9), sodium tetradecyl sulfate (10), and 20% hypertonic saline (11).
Ethanol has generally been the most widely used sclerosing agent in clinical practice, but its effectiveness is decreased through dilution by the fluid remaining in the renal cyst. The mean Hounsfield value of 99.9% ethanol did decrease to -190 Hounsfield units (HU) and there was a linear correlation between ethanol concentration and Hounsfield values in our previous study (5). This suggests that monitoring of the HU of ethanol by computed tomography (CT) may be an effective method in ethanol sclerotherapy of renal cysts. According to the previously available data, percutaneous treatment for simple renal cysts with ethanol concentration monitoring has not been fully evaluated, yet. Therefore, we conducted this exploratory randomized trial to evaluate whether CT-guided sclerotherapy with ethanol concentration monitoring yields better results than without ethanol concentration monitoring in the treatment of simple renal cysts. We also describe improvements in the method according to previously available data and practices.
In a 12-month exploratory randomized trial, treatment of simple renal cysts by sclerotherapy with ethanol concentration monitoring was compared with that without ethanol concentration monitoring. Randomization was performed using a systematic sampling technique.
All patients with a simple renal cyst who were admitted to our hospital between May 2009 and September 2011 were considered candidates for the study.
Informed consent was obtained from all patients before sclerotherapy. The study was approved by the institutional review board and ethics commission. The inclusion criteria included patients who presented with symptoms and signs caused by a simple renal cyst or patients in whom the cyst size had increased. The exclusion criteria included previously treated renal cysts except for treatment with oral analgesics; infectious renal cyst; abnormal renal function; communication between the renal collecting system and simple renal cysts; polycystic or cystic dysplastic kidneys; renal cysts associated with tuberous sclerosis or previous malignancy; peripelvic renal cysts; and cyst size < 2 cm.
Eligible patients were randomly assigned into two groups for either sclerotherapy without ethanol concentration monitoring (group A) or sclerotherapy with ethanol concentration monitoring (group B). In patients who had more than one cyst, each was separately documented. A simple renal cyst was diagnosed by ultrasound or CT according to the criteria of Bosniak (12). Cyst volume was calculated by applying the formula V = π / 6 (length × width × depth). The maximum diameters of the cysts were measured.
All sclerotherapies were guided by a single slice helical CT scanner (Xvision, Toshiba, Tochigi, Japan). The scanning parameters of the scanner were 120 kVp, 200 mA, 5 mm collimation, and 5 mm slice thickness. The window width and level were approximately 250 and 60 HU, respectively. The patients were placed in prone positions and were instructed to breathe calmly during the procedure. CT images were obtained through the area of the cysts using a section thickness of 5 mm. Localization was determined by CT gantry laser lights and a landmark using a self-made radiopaque grid on the patient's skin. At the site of puncture, approximately 3 mL of 1% xylocaine was injected subcutaneously as local anesthesia. After the cyst was punctured using a 19-gauge coaxial needle (TruGuide, Bard, Tempe, AZ, USA) under CT guidance, the needle core was removed and a three-way stopcock was connected to the needle. Cystic fluid was aspirated through the needle as much as possible. The volume of the aspirated fluid was recorded. The fluid sample was sent for bacteriological, cytological and biochemical (lipid, protein, lactate dehydrogenase) examinations. Diluted contrast medium (Iohexol 300 mg I/mL, Yangtze River, Jiangsu, China) was injected into the cyst (15% of the aspirated volume) under CT guidance to ensure that there was no communication of the cyst with the collecting system or leakage into the peritoneal cavity. After confirming a smooth margin and no extravasation, the contrast medium was completely drained.
In group A, the contrast medium was replaced with 99.9% ethanol, corresponding to 25% of the volume of the aspirated fluid. The maximum volume of ethanol injected was 200 mL to avoid systemic side effects, irrespective of the size of the cyst. The patient was placed in the prone and bilateral decubitus positions at least one time during 20 minutes in order to achieve satisfactory contact between alcohol and all surfaces of the cyst cavity (1, 6, 7, 11). Then the ethanol injected was completely removed and complete deflation of the cyst cavity was verified by CT.
In group B, the first injection of the 99.9% ethanol solution was equivalent to 25% of the aspirated cyst fluid but not more than 200 mL in a single injection. Then the repeated aspiration and injection were performed with 10-20 mL in each injection. Generally, the total number of repeated aspirations and injections was about 2-8 according to the size of the cyst. The amount of ethanol in a cyst was always maintained at a volume equivalent to 25% of the aspirated fluid in this way. When the aspiration fluid was clear and colorless, CT was performed to confirm the Hounsfield value. Considering the ionizing radiation exposure, we only chose the maximum section of the lesion to scan. If the Hounsfield value of the cyst fluid reached -190 HU, the patient was placed in the prone and bilateral decubitus positions at least one time during 10 minutes in order to achieve satisfactory contact between the alcohol and all surfaces of the cyst cavity. Then the ethanol injected was withdrawn (Fig. 1).
To ensure patient safety, we checked the vital signs every 5-10 minutes during the procedure; therefore, the ethanol concentration in the blood was not measured routinely. After the procedure, all patients were observed in the care units for at least 2 hours, and patients without complications were discharged.
Technical success was defined as achievement of complete aspiration and sclerotherapy. Patients were followed every 3 months for 1 year by clinical assessment and ultrasonography. The maximum diameter of the cyst was measured and compared with the primary diameter. Disappearance of the renal cyst with no symptoms was considered to be complete regression. A more than 50% reduction in the size with relief of symptoms was considered partial regression. Size reductions of less than 50% and/or persistent symptoms were considered treatment failure.
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). We used the chi-square test for categorical variables and the independent t test and Mann-Whitney U test for continuous variables to compare differences in the response between the two groups. To evaluate whether the findings were stable against the possible influence of other variables, we performed a multivariate logistic regression, and variables related to age, gender, cyst laterality, cyst size, treatment group and volume of aspirated fluid were selected. P values less than 0.05 were considered statistically significant.
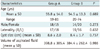
Simple renal cysts were diagnosed in 72 patients during the study period. Five of them were excluded because three patients refused to participate, and two patients had peripelvic renal cysts. Of the remaining 67 patients with 70 simple renal cysts, the indications for the procedure were flank pain in 55 patients, patient reassurance due to increasing cyst size in 12; 33 patients with 35 cysts received sclerotherapy without ethanol concentration monitoring in group A, while 34 patients with 35 cysts underwent sclerotherapy with ethanol concentration monitoring in group B (Fig. 2). There were 32 men and 35 women, with a mean age of 54.8 ± 13.6 years (range: 19-81). Characteristics of the patient demographics, renal cysts and procedures are shown in Table 1.
The sclerotherapy treatments were technically successful in all 67 patients. In all cyst fluids, there was no abnormality on bacteriologic, cytologic, or biochemical examinations. The mean aspirated volume of the 70 renal cysts was 391.6 mL. In group A, the mean was 398.8 mL with a range of 75-1080 mL; in group B, the mean was 384.4 mL with a range of 70-1090 mL. There were no differences in patient characteristics, mean cyst size and aspirated volume of cysts between the 2 groups (p > 0.05) (Table 1). The mean ethanol exposure time was 18.7 minutes in group B, which was less than that in group A (p = 0.005) (Table 2).
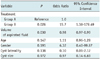
During the 12-month follow-up period, complete regression, partial regression and treatment failure occurred in 20, 6 and 9 cysts, respectively, in group A, and 29, 4 and 2 cysts, respectively, in group B. The overall success rate (complete regression and partial regression) was 74.3% (26 of 35 cysts) in group A and 94.3% (33 of 35 cysts) in group B (p = 0.022) (Table 2). The mean cyst size before and after treatment were 8.6 ± 2.0 cm and 2.3 ± 2.9 cm, respectively, in group A, and 8.4 ± 1.7 cm and 0.8 ± 1.9 cm, respectively, in group B (Tables 1, 2) (Figs. 3, 4). In group A, 21 of 25 (84%) symptomatic patients had relief after therapy, and in group B, clinical symptoms resolved in 28 of 30 (93.3%) symptomatic patients. None of the cysts recurred after disappearance. The success of cyst regression was correlated with treatment. Treatment with ethanol concentration monitoring was approximately 16 times more effective than that without ethanol concentration monitoring (p = 0.026; odds ratio = 15.7; 95% confidence interval: 1.38-179.49) (Table 3). The volume of the aspirated fluid influenced the success rate, i.e., the larger the aspiration volume, the smaller the chance of treatment success (p = 0.030). The other variables did not influence outcome.
There were no major complications. In groups A and B, pain at injection sites occurred in 5 and 7 cases, respectively; fever occurred in 1 and 3 cases, respectively (Table 2). In both groups, symptoms such as fever or pain did not require medical management and disappeared within 1-2 days.
Most simple renal cysts are asymptomatic and do not have any impact on renal function. These simple cysts usually are managed conservatively. However, treatment may be required if flank or back pain, hypertension, infection, deterioration of renal function or renal outflow obstruction occur. With new technical advances in interventional radiology, the recent trend in the treatment of symptomatic renal cysts has been more strongly toward minimally invasive methods in the form of percutaneous treatment (13).
Percutaneous aspiration is a simple and safe procedure that has been increasingly reported in recent years. However, simple drainage without sclerotherapy is associated with a recurrence rate of 30-80% (1, 14). Because the cyst wall epithelium is responsible for active liquid production, it must be destroyed in order to prevent recurrent disease. The percutaneous approach using a sclerosing agent provides more satisfactory results than aspiration alone (1). So, percutaneous sclerotherapy is usually used as the first-line treatment before surgical and laparoscopic methods because of its minimally invasive nature. Several sclerosing agents like ethanol, polidocanol, glucose, acetic acid, OK-432, ethanolamine oleate, sodium tetradecyl sulfate and 20% hypertonic saline have been used to injure the epithelial cells of the cyst wall (1-11). Ethanol has been commonly used for sclerosing renal cysts. The efficacy of ethanol sclerotherapy can vary with regard to the time of exposure to ethanol, the volume of ethanol, the number of sclerotherapy sessions and so on. This study, to our knowledge, represents the first prospective randomized trial comparing the differences between sclerotherapy with and without ethanol concentration monitoring for the treatment of simple renal cysts.
In the majority of studies involving ethanol sclerotherapy, although those researchers reported favorable therapeutic outcomes with varying methods, optimal methods of treating renal cysts with reasonable cost-effectiveness have not yet been established. Generally, most authors used from 20 to 30 minutes exposure time to ethanol in single-session sclerotherapy (1, 5-8, 11, 13). In our study, the mean ethanol exposure time was 18.7 minutes in group B, which was less than that in group A. Lin et al. (15) reported that there was no statistical difference in therapeutic efficacy between 2- and 4-hour ethanol-retention techniques. Because no data on ethanol concentration versus retention time in renal cysts were available in their study, they hypothesized that two factors might have contributed to the result. First, nearly all the epithelial cells on the cyst was destroyed after 2 hours of ethanol retention. Second, the concentration of ethanol in the cyst after the 2-hour retention was diluted to below the level of the coagulating effect on the cyst epithelium. The mean Hounsfield value of 99.9% ethanol went down to -190 HU (5). In our study, we observed that the aspirated cyst fluid was clear at the beginning, but the aspirated liquid became cloudy after the injection of ethanol. The Hounsfield value of the aspirated cystic fluid only measured between -100 and 20 HU. Therefore, in group B, if the Hounsfield value of the cyst fluid was not as low as -190 HU, then repeated aspiration and injection were performed, with about 10-20 mL in each injection. So, during the 12-month follow-up period, the overall success rate was significantly better in group B (94.3%) than in group A (74.3%) (p = 0.022).
The high success rate in group B might be attributed to several reasons. First, the use of a three-way stopcock connected with the needle prevented air from entering the renal cyst, allowing the ethanol to completely contact all the epithelial cells on the cyst. Second, the repeated aspirations and injections were beneficial for eliminating the dilution of ethanol. Finally, sclerotherapy with ethanol concentration monitoring assured the presence of a high concentration of ethanol (99.9%) to inactivate the epithelial cells on the renal cyst. Our results indicated that the likelihood of treatment with ethanol concentration monitoring being successful was approximately 16 times higher than that without ethanol concentration monitoring.
Some authors (1, 8, 16) have used a multiple-session technique with a 12-hour to 48-hour interval between sessions. These results suggest that multiple sclerotherapy is better than a single injection of sclerosant for reducing the recurrence of simple renal cysts. High success rates of single-session aspiration and sclerotherapy have been reported (5, 6, 11). Based on those results, we chose sclerotherapy with ethanol concentration monitoring during a single-session sclerotherapy procedure as the primary treatment of simple renal cysts. In our study, the 94.3% success rate in group B was significantly better compared with previous reports (1, 6, 8, 11, 16).
The volume of ethanol injected after aspiration varies from 15% to 75% of the cyst volume, the maximum volume being 100-200 mL in various reports (5, 7, 8, 11, 16). The concentration of ethanol applied varied from 95 to 99.9%, but no author has been able to demonstrate any difference in the results related to these variations (1, 5, 7, 8, 11, 13, 16).
There were several limitations to our study. One limitation included a radiation hazard of this study compared with US-guided procedures. Another limitation involved the relatively small sample sizes of patients receiving sclerotherapy with ethanol concentration monitoring, and further randomized studies with larger patient groups are required to confirm our results. Another limitation was that all patients had only a 12-month follow-up period. The overall success rate may have been affected by the difference in follow-up length between the two groups.
In conclusion, this study showed that monitoring of the HU of ethanol by CT is an effective method in ethanol ablation of renal cysts. The HU (-190) of the CT scan corresponded to the point of clear fluid aspiration; therefore, the ethanol ablation procedure could be terminated at the point of clear fluid aspiration.
Figures and Tables
Fig. 1
69-year-old woman with persistent flank pain for 6 months.
(A) Simple renal cyst before sclerotherapy; (B) diluted contrast medium (15% of aspirated volume) was injected into cyst to ensure that there was no communication of cyst with collecting system or leakage into peritoneal cavity; and (C) contrast medium was replaced with 99.9% ethanol in amount corresponding to 25% of volume of aspirated fluid. After case underwent repeated aspirations and injections, with 20 mL in each injection, for five times, Hounsfield value of cyst fluid reached -201 HU; and (D) ethanol injected was withdrawn. HU = Hounsfield units
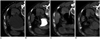
Fig. 3
Cyst size before and after treatment in group A. Mean cyst size before and after treatment was 8.6 ± 2.0 cm and 2.3 ± 2.9 cm (p < 0.001).

Fig. 4
Cyst size before and after treatment in group B. Mean cyst size before and after treatment was 8.4 ± 1.7 cm and 0.8 ± 1.9 cm (p < 0.001).

References
1. Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: value of two injections of a sclerosing agent. AJR Am J Roentgenol. 1996; 167:781–783.
2. Okeke AA, Mitchelmore AE, Keeley FX, Timoney AG. A comparison of aspiration and sclerotherapy with laparoscopic de-roofing in the management of symptomatic simple renal cysts. BJU Int. 2003; 92:610–613.
3. Sandler CM, Houston GK, Hall JT, Morettin LB. Guided cyst puncture and aspiration. Radiol Clin North Am. 1986; 24:527–537.
4. Agarwal M, Agrawal MS, Mittal R, Sachan V. A randomized study of aspiration and sclerotherapy versus laparoscopic deroofing in management of symptomatic simple renal cysts. J Endourol. 2012; 26:561–565.
5. Xu XX, Du Y, Yang HF, Zhang Q, Li Y, Zee CS. CT-guided sclerotherapy with ethanol concentration monitoring for treatment of renal cysts. AJR Am J Roentgenol. 2011; 196:W78–W82.
6. Akinci D, Akhan O, Ozmen M, Gumus B, Ozkan O, Karcaaltincaba M, et al. Long-term results of single-session percutaneous drainage and ethanol scleroth erapy in simple renal cysts. Eur J Radiol. 2005; 54:298–302.
7. Cho DS, Ahn HS, Kim SI, Kim YS, Kim SJ, Jeon GS. Sclerotherapy of renal cysts using acetic acid: a comparison with ethanol sclerotherapy. Br J Radiol. 2008; 81:946–949.
8. Ham WS, Lee JH, Kim WT, Yu HS, Choi Y. Comparison of multiple session 99% ethanol and single session OK-432 sclerotherapy for the treatment of simple renal cysts. J Urol. 2008; 180:2552–2556.
9. Yamamoto K, Sakaguchi H, Anai H, Tanaka T, Morimoto K, Kichikawa K, et al. Sclerotherapy for simple cysts with use of ethanolamine oleate: preliminary experience. Cardiovasc Intervent Radiol. 2005; 28:751–755.
10. Demir E, Alan C, Kilciler M, Bedir S. Comparison of ethanol and sodium tetradecyl sulfate in the sclerotherapy of renal cyst. J Endourol. 2007; 21:903–905.
11. Egilmez H, Gok V, Oztoprak I, Atalar M, Cetin A, Arslan M, et al. Comparison of CT-guided sclerotherapy with using 95% ethanol and 20% hypertonic saline for managing simple renal cyst. Korean J Radiol. 2007; 8:512–519.
12. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986; 158:1–10.
13. Zerem E, Imamovíc G, Omerovíc S. Symptomatic simple renal cyst: comparison of continuous negative-pressure catheter drainage and single-session alcohol sclerotherapy. AJR Am J Roentgenol. 2008; 190:1193–1197.
14. Stevenson JJ, Sherwood T. Conservative management of renal masses. Br J Urol. 1971; 43:646–647.
15. Lin YH, Pan HB, Liang HL, Chung HM, Chen CY, Huang JS, et al. Single-session alcohol-retention sclerotherapy for simple renal cysts: comparison of 2- and 4-hr retention techniques. AJR Am J Roentgenol. 2005; 185:860–866.
16. Chung BH, Kim JH, Hong CH, Yang SC, Lee MS. Comparison of single and multiple sessions of percutaneous sclerotherapy for simple renal cyst. BJU Int. 2000; 85:626–627.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download