Abstract
Objective
To evaluate the upgrade rate and delayed false-negative results of percutaneous vacuum-assisted removal (VAR) and surgical excision in women with imaging-histologic discordance during ultrasound (US)-guided automated core needle biopsy (CNB) of the breast and to validate the role of VAR as a rebiopsy method for these discordant lesions.
Materials and Methods
Percutaneous US-guided 14-gauge CNB was performed on 7470 patients between August 2005 and December 2010. Our study population included 161 lesions in 152 patients who underwent subsequent rebiopsy due to imaging-histologic discordance. Rebiopsy was performed using VAR (n = 88) or surgical excision (n = 73). We investigated the upgrade rate immediately after rebiopsy and delayed false-negative results during at least 24 months of follow-up after rebiopsy. We also evaluated the clinicoradiological differences between VAR and surgical excision.
Results
Total upgrade to malignancy occurred in 13.7% (22/161) of lesions at rebiopsy including both VAR and surgical excision: 4.6% (4/88) of VAR cases (4/88) and 24.7% (18/73) of surgical excision cases (p < 0.001). Surgical excision was performed significantly more frequently in older patients and for larger-sized lesions than that of VAR, and a significant difference was detected between VAR and surgical excision in the Breast Imaging and Reporting and Data System category (p < 0.007). No delayed false-negative results were observed after VAR or surgical excision during the follow-up period.
Ultrasound (US)-guided core needle biopsy (CNB) is an accurate and standard method for sampling lesions that are visible on US (1). However, CNB may underestimate results (2, 3) or produce false-negative results (4, 5), and various factors can affect CNB accuracy (6). Therefore, many solutions have been suggested, including confirmation of lesion retrieval by specimen radiography, correlating post-biopsy imaging and histology, larger sampling for imaging-histologic discordant lesions, and follow-up after benign biopsy results (7).
The frequency of imaging-histologic discordance has been reported to be 1-6%, and several studies have reported imaging-histologic discordance following stereotactic CNB (4, 8, 9) and US-guided CNB (1, 6, 8, 10, 11, 12). These reports show malignancy rates of 6.8-29.3% in imaging-histologic discordant lesions during US-guided biopsy (1, 6, 10, 12, 13, 14, 15), suggesting that rebiopsy of those lesions will yield benign pathology in approximately 70.7-93.2% of cases. Considering the large percentage of benign pathology at rebiopsy, Kim et al. (12) used vacuum-assisted removal (VAR) on discordant cases for a definitive diagnosis. However, the number of patients in that study was small (n = 26), and the follow-up period was limited. Some investigators have reported results of VAR management of imaging-histologic discordant lesions (13, 15). However, the number of patients in those studies was small (n = 28 and 55), and they used a 16-gauge core biopsy needle instead of a 14-gauge core biopsy needle (13, 15). Moreover, their follow-up period was 12 months, which is insufficient to confirm the absence of false-negative biopsy results after VAR.
Therefore, the purpose of this study was to evaluate the upgrade rate and delayed false-negative results of percutaneous VAR in women with imaging-histologic discordance during US-guided automated CNB of the breast and to compare these results with those of surgical excision.
This retrospective study was approved by our Institutional Review Board, and informed consent was waived.
Percutaneous US-guided 14-gauge CNB was performed on 7470 female patients between August 2005 and December 2010. US-guided CNB was performed using a free-hand technique, guided by a 5-10 MHz or 5-12 MHz linear-array transducer (ATL HDI 5000 or iU-22 Philips Advanced Technology Laboratories, Bothell, WA, USA). A 14-gauge semi-automated CNB (Stericut, TSK Laboratory, Tochigi, Japan) was used. All biopsies were performed by one of six full-time board-certified radiologists dedicated to breast imaging. The radiologists had varying degrees of experience in breast imaging of 1-10 years. Typically, four or five core samples were obtained, and the number of core samples was determined at the discretion of each radiologist.
All breast-dedicated radiologists reviewed the final pathological reports for all biopsied lesions on a weekly basis. These reports were reviewed in conjunction with mammographic and sonographic images within 1 week of biopsy. Radiologists determined whether the imaging findings and histological results were concordant or discordant. The imaging and histological findings were considered "concordant" when the histological findings provided an acceptable explanation for the imaging features and "discordant" when they did not (12). Discordant lesions included: 1) lesions that were suspicious for malignancy, e.g., Breast Imaging and Reporting and Data System (BI-RADS) category 4 (16), when the histological findings did not account for the imaging features (12); and 2) lesions that were highly suggestive of malignancy, e.g., BI-RADS category 5, revealing benign histological results, unless the specific histology accounted for the imaging features. Findings were considered "concordant" after a consensus was obtained among all the radiologists on each case. However, if at least one radiologist disagreed, the lesion was regarded as "discordant".
Vacuum-assisted removal (Mammotome; Ethicon Endo Surgery, Cincinnati, OH, USA) was recommended for discordant lesions (12). VAR was performed after administering local anesthesia. The gauge of the probe used was determined by the lesion size: an 8-gauge probe for lesions > 1.5 cm, an 11-gauge probe for lesions < 1.0 cm, and an 8-gauge or 11-gauge probe for lesions 1.0-1.5 cm in the largest dimension, according to the patient's breast thickness after a small skin incision. Multiple core samples were obtained under US guidance until the mass was completely removed. Skin tears or chest wall muscle injuries were regarded as serious complications (12). A US image was taken after biopsy to verify that the mass had been removed completely.
Some lesions were surgically excised, and this decision was made at the discretion or request of the physician or patient. Thus, 265 lesions in 250 patients were assessed as imaging-histologic discordant results after the imaging-histologic correlation was performed. Among all lesions, we excluded 66 lesions that had undergone imaging follow-up without rebiopsy, such as VAR or surgical excision, at our institution. We also excluded 38 lesions in which the histological findings mandated a surgical excision regardless of the US findings, including papilloma (n = 13), atypical ductal hyperplasia (n = 3), phyllodes tumor (n = 1), and discordant malignant lesions (n = 21). Therefore, our study population included 152 patients with 161 lesions (Fig. 1). Of the patients who underwent VAR and surgical excision, 97 (64%, 97/152) were followed for more than 24 months (median, 43 months; range, 24-88 months). Patients with less than 24 months of follow-up after VAR (n = 36) or surgical excision (n = 19) were contacted by phone to ask about breast cancer occurrence thereafter.
The lesions were classified into the final US BI-RADS assessment categories prior to US-guided CNB (16). Surgical excision or follow-up US, imaging studies, and histological findings were reviewed, and follow-up data were obtained for breast lesions with imaging-histologic discordance at the initial percutaneous 14-gauge CNB and that underwent subsequent VAR.
Age, BI-RADS category, and histological results were reviewed from medical records. We calculated the discordant lesion upgrade rates for VAR and surgical excision. Upgrade was defined when a patient had at least one lesion classified as a carcinoma at VAR or surgical excision. The upgrade rate was assigned to patients with ductal carcinoma in situ or invasive carcinoma. We also investigated delayed false-negative results during the follow-up period after VAR or surgical excision. Statistical analyses were performed using the chi-square test for categorical variables and the t test for continuous variables. The data were processed with the SPSS ver. 20.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Among the 161 lesions in 152 patients who had discordant results after the imaging-histologic correlation, 73 lesions were removed by surgical excision and 88 lesions were removed by VAR. All 152 patients were female with a median age of 45 years (range, 19-77 years; mean, 45.4 years). Among the 88 breast lesions with VAR, 62 were biopsied with an 8-gauge probe, and 26 lesions were biopsied with an 11-gauge probe. The median number of acquired tissue samples was 17 (range, 6-176). No serious complications were observed after VAR.
Older patients were referred to surgical excision (mean ± standard deviation [SD], 47.9 ± 10.1 years) more often than VAR (mean ± SD, 43.2 ± 10.9 years) (p = 0.007) (Table 1). Additionally, the lesions that were surgically excised were larger than the lesions that received VAR (16.9 ± 10.8 mm vs. 13.6 ± 6.3 mm) (p = 0.021). A significant difference was observed in the BI-RADS category for VAR and surgical excision (p = 0.026); surgical excision was performed more often for lesions in categories 4c and 5 compared to VAR.
Table 2 shows the initial histological results that included imaging-histologic discordant lesions (n = 161). Twenty-two lesions (VAR 4.6%, 4/88; surgical excision 24.7%, 18/73) were upgraded to malignant after rebiopsy (Figs. 2, 3). A significant difference in the upgrade rate was observed between VAR and surgical excision (p < 0.001). The histological profiles of initial CNB, VAR, and surgical excision in the upgraded lesions (n = 22) are listed in Table 3.
Ultrasound-guided CNB is an easily accessible and accurate method to diagnose breast lesions. However, underestimated rates and false-negative results could be a drawback of US-CNB as a biopsy method (2, 3, 4, 5). Therefore, the imaging-histologic correlation after US-CNB is widely used as a method to reduce the false-negative and underestimated results of CNB. Additionally, larger samples are required for imaging-histologic discordant lesions, so surgical excision has traditionally been used to biopsy such lesions (10). However, VAR was recently reported as an alternative to surgery because of its efficacy (12, 13, 15, 17, 18). Rajan et al. (18) reported adopting vacuum-assisted biopsy (VAB) instead of diagnostic surgery for lesions of uncertain malignant potential, such as radial scars, papillomas, flat epithelial atypia, atypical ducal hyperplasia, and atypical lobular hyperplasia, to reduce the number of benign-diagnosed surgical biopsies, suggesting VAB as an alternative biopsy method to surgery. We focused only on the VAR indication in cases of imaging-histologic discordance after 14-gauge CNB but we did not include high risk lesions, as in Rajan et al. (18). Many studies (1, 6, 9, 10, 12, 13, 15, 19) have reported the role of VAR; however, they included all VAR cases, not just those that were imaging-histologic discordant. Son et al. (1) performed VAR in 53 lesions among 103 imaging-histologic discordant cases, but we included a larger study population with discordant results (1).
We evaluated the difference in upgrade rates between VAR and surgical excision and the role of VAR as a rebiopsy method for imaging-histologic discordant lesions after US-guided CNB with long-term follow-up results. The upgrade rate in the imaging-histologic discordant lesions after VAR or surgical excision has been reported to be 6.8-29.3% (Table 4) (1, 6, 10, 12, 13, 14, 15). The upgrade rates in our study were 4.6% and 24.6% for VAR and surgical excision, respectively, which is similar to the range reported previously. Surgical excision was performed significantly more frequently on older patients, with larger lesions, and on category 4c and 5 lesions compared to those of VAR. These results suggest that the rebiopsy method was determined by the surgeon's discretion with patient preference limited in scope. The most significant reason for the differences between VAR and surgical excision was that surgeons directly performed therapeutic surgery after confirming the frozen excisional biopsy result for more suspicious lesions rather than VAR. Our upgrade rate was 13.6% in all lesions; however, if all lesions were surgically excised, unnecessary surgery could reach up to 86.4%. Therefore, VAR could be an alternative method for reducing needless surgery and planning a well-designed operation. Kim et al. (12) also reported that surgical excision was performed more frequently on BI-RADS category 4c lesions compared to VAR, compatible with our result. However, that study reported no difference between VAR and surgical excision, unlike our study (12).
Liberman et al. (10) reported that 10.5% (2/19) of their results were immediate false negatives under US-guidance during a 7-year period. Their upgrade rate to malignancy (10.5%) was higher than our upgrade rate (4.6%), though these two results are almost within the previously reported range (6.8-29.3%) (1, 6, 10, 12, 13, 14, 15). Imaging-histologic discordance tends to be determined by the radiologist's experience rather than by well-established criteria. However, the upgrade rates of these discordant lesions were still greater than the reported 2% probability of malignancy in BI-RADS category 3, which are primarily benign lesions. Thus, larger tissue samples are required for imaging-histologic discordant lesions. Appropriate follow-up should be performed after benign biopsy results to prevent immediate and delayed false-negative results.
No delayed cancer diagnoses were made in either the follow-up patients or patients contacted by phone in the present study. A few studies have reported delayed false-negative results (20, 21, 22, 23), Peter et al. (21) reported a 1.4% rate of delayed false-negative results; however, their results were obtained by stereotactically guided vacuum biopsy, instead of US-guided VAR; thus, they could not warrant complete removal of the lesions. Unlike US-guidance, it is difficult to completely remove lesions with stereotactic VAB because lesion boundaries cannot be assessed during the procedure, which may explain their delayed false-negative results. Perez-Fuentes et al. (20) reported no delayed false-negative results after US-guided 11-gauge VAB. Although their complete removal rate was limited to 88.6% (78/88 lesions) and 24-month follow-up data were available for 71 lesions, their study was not confined to imaging-histologic discordant lesions. Two other studies (22, 23) reported no delayed false-negative results after VAR for benign lesions. However, their indication was benign lesions; thus, delayed false-negative results were not within the scope of those studies. Therefore, the current study is the first to report a lack of VAR delayed false-negative results in imaging-histologic discordant lesions with a large number of patients and a long-term follow-up.
Our study had several limitations. First, the VAR and surgery groups were not randomized due to the retrospective nature of the study and were dissimilar in major patient characteristics, as the rebiopsy method was determined according to the surgeon's discretion. The most significant reason for the differences between VAR and surgical excision was that the surgeon directly performed the therapeutic surgery after confirming the frozen excisional biopsy result for more suspicious lesions rather than VAR. Although our results suggest applying VAR more broadly, a prospective randomized study will be necessary to solve this limitation. Second, some patients who did not have sufficient imaging follow-up data, including those with less than a 24 month follow-up, were contacted by phone regarding the occurrence of breast cancer after VAR (n = 36) or surgical excision (n = 19). These patient's responses were not verified by imaging follow-up; however, the phone interviews were performed more than 2 years after VAR or surgical excision. Therefore, no cancer occurrence after more than 2 years was evident.
In conclusion, long-term follow-up data showed no delayed cancer diagnoses after US-guided VAR in imaging-histologic discordant lesions of the breast; thus, VAR might be considered a rebiopsy method for imaging-histologic discordant lesions.
Figures and Tables
Fig. 2
51-year-old woman with mammographic abnormality.
A. Left mediolateral oblique mammography view shows partially obscured hyperdense mass. B. Transverse sonogram corresponding to mammographic abnormality shows microlobulated hypoechoic mass (arrows) classified as Breast Imaging and Reporting and Data System category 4a. Ultrasound-guided core needle biopsy reveals fibrocystic change; however, this result was regarded as discordant benign. C. Eleven-gauge vacuum-assisted removal (arrowheads) was performed, and histological results indicated invasive ductal carcinoma.
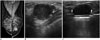
Fig. 3
77-year-old woman with palpable mass in her left breast.
A. Left mediolateral oblique mammography view shows irregular shaped hyperdense mass. B, C. Transverse (B) and longitudinal (C) sonogram corresponding to palpable site shows irregular and hypoechoic mass (arrows) classified as Breast Imaging and Reporting and Data System category 5. Ultrasound-guided core needle biopsy revealed intraductal papilloma; however, this result was regarded as discordant benign. Surgical excision was performed, and histological results revealed ductal carcinoma in situ.
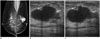
Table 1
Age, Lesion size, BI-RADS Categories According to Tissue Acquisition Method in Imaging-Histologic Discordant Lesions (n = 161)
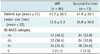
Table 2
Initial Histologic Results of Imaging-Histologic Discordant Lesions (n = 161)

| Initial CNB Results | Frequency |
|---|---|
| Fibrocystic change | 53 |
| Stromal fibrosis | 40 |
| Fibroadenoma | 18 |
| Adenosis | 16 |
| Fibroadenomatous hyperplasia | 14 |
| Ductal epithelial hyperplasia | 11 |
| Duct ectasia | 5 |
| Other* | 4 |
| Total | 161 |
Table 3
Frequency of Carcinoma after VAR or Surgical Excision in Imaging-Histologic Discordant Lesions (n = 22)
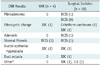
Table 4
Published Reports Applying of Vacuum-Assisted Biopsy or Removal to Imaging-Histologic Discordant Lesions

| Investigator | Rebiopsy Method | Population* | Upgrade to Malignancy (%) | Follow-Up Period |
|---|---|---|---|---|
| Kim et al. (12) | US-VAR (8-G, 11-G) | 18 | 11.1 | NS |
| Li et al. (13) | US-VAB (10-G) | 28 | 21.4 | 1 year |
| Wang et al. (15) | US-VAB or VAR (7-G) | 55 | 22.7 (VAR) | 1 year |
| 21.2 (VAB) | ||||
| Our study | US-VAR (8-G, 11-G) | 88 | 4.6 | At least 2 year |
Notes
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (grant 2013R1A1A3013165) and by a faculty research grant of Yonsei University College of Medicine for 2013 (6-2013-0094).
References
1. Son EJ, Kim EK, Youk JH, Kim MJ, Kwak JY, Choi SH. Imaging-histologic discordance after sonographically guided percutaneous breast biopsy: a prospective observational study. Ultrasound Med Biol. 2011; 37:1771–1778.
2. Jang M, Cho N, Moon WK, Park JS, Seong MH, Park IA. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2008; 191:1347–1351.
3. Suh YJ, Kim MJ, Kim EK, Moon HJ, Kwak JY, Koo HR, et al. Comparison of the underestimation rate in cases with ductal carcinoma in situ at ultrasound-guided core biopsy: 14-gauge automated core-needle biopsy vs 8- or 11-gauge vacuum-assisted biopsy. Br J Radiol. 2012; 85:e349–e356.
4. Shah VI, Raju U, Chitale D, Deshpande V, Gregory N, Strand V. False-negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 concecutive cases of missed breast cancer. Cancer. 2003; 97:1824–1831.
5. Youk JH, Kim EK, Kim MJ, Kwak JY, Son EJ. Analysis of false-negative results after US-guided 14-gauge core needle breast biopsy. Eur Radiol. 2010; 20:782–789.
6. Kim MJ, Kim EK, Park SY, Jung HK, Park BW, Kim H, et al. Imaging-histologic discordance at sonographically guided percutaneous biopsy of breast lesions. Eur J Radiol. 2008; 65:163–169.
7. Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics. 2007; 27:79–94.
8. Meyer JE, Smith DN, Lester SC, DiPiro PJ, Denison CM, Harvey SC, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998; 206:717–720.
9. Philpotts LE, Shaheen NA, Carter D, Lange RC, Lee CH. Comparison of rebiopsy rates after stereotactic core needle biopsy of the breast with 11-gauge vacuum suction probe versus 14-gauge needle and automatic gun. AJR Am J Roentgenol. 1999; 172:683–687.
10. Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000; 89:2538–2546.
11. Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998; 208:717–723.
12. Kim MJ, Kim EK, Lee JY, Youk JH, Park BW, Kim SI, et al. Breast lesions with imaging-histologic discordance during US-guided 14G automated core biopsy: can the directional vacuum-assisted removal replace the surgical excision? Initial findings. Eur Radiol. 2007; 17:2376–2238.
13. Li JL, Wang ZL, Su L, Liu XJ, Tang J. Breast lesions with ultrasound imaging-histologic discordance at 16-gauge core needle biopsy: can re-biopsy with 10-gauge vacuum-assisted system get definitive diagnosis? Breast. 2010; 19:446–449.
14. Mihalik JE, Krupka L, Davenport R, Tucker L, Toevs C, Smith RS. The rate of imaging-histologic discordance of benign breast disease: a multidisciplinary approach to the management of discordance at a large university-based hospital. Am J Surg. 2010; 199:319–323. discussion 323.
15. Wang ZL, Liu G, Li JL, Su L, Liu XJ, Wang W, et al. Breast lesions with imaging-histologic discordance during 16-gauge core needle biopsy system: would vacuum-assisted removal get significantly more definitive histologic diagnosis than vacuum-assisted biopsy? Breast J. 2011; 17:456–461.
16. American college of radiology. Breast imaging reporting and data system (BI-RADS) ultrasound. 4th ed. Reston, VA: American College of Radiology;2003.
17. Cassano E, Urban LA, Pizzamiglio M, Abbate F, Maisonneuve P, Renne G, et al. Ultrasound-guided vacuum-assisted core breast biopsy: experience with 406 cases. Breast Cancer Res Treat. 2007; 102:103–110.
18. Rajan S, Shaaban AM, Dall BJ, Sharma N. New patient pathway using vacuum-assisted biopsy reduces diagnostic surgery for B3 lesions. Clin Radiol. 2012; 67:244–249.
19. Dershaw DD, Morris EA, Liberman L, Abramson AF. Nondiagnostic stereotaxic core breast biopsy: results of rebiopsy. Radiology. 1996; 198:323–325.
20. Perez-Fuentes JA, Longobardi IR, Acosta VF, Marin CE, Liberman L. Sonographically guided directional vacuum-assisted breast biopsy: preliminary experience in Venezuela. AJR Am J Roentgenol. 2001; 177:1459–1463.
21. Peter D, Grünhagen J, Wenke R, Schäfer FK, Schreer I. False-negative results after stereotactically guided vacuum biopsy. Eur Radiol. 2008; 18:177–182.
22. Kim MJ, Park BW, Kim SI, Youk JH, Kwak JY, Moon HJ, et al. Long-term follow-up results for ultrasound-guided vacuum-assisted removal of benign palpable breast mass. Am J Surg. 2010; 199:1–7.
23. Li S, Wu J, Chen K, Jia W, Jin L, Xiao Q, et al. Clinical outcomes of 1,578 Chinese patients with breast benign diseases after ultrasound-guided vacuum-assisted excision: recurrence and the risk factors. Am J Surg. 2013; 205:39–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download