Abstract
Mycobacterium szulgai (M. szulgai) is an unusual pathogen in a human non-tuberculous mycobacterial infection. Pulmonary infection due to M. szulgai may be clinically and radiologically confused with active pulmonary tuberculosis. In contrast to other non-tuberculous mycobacteria, M. szulgai infection is well controlled by combination antimycobacterial therapy. Most of the previously reported cases of M. szulgai pulmonary infection showed cavitary upper lobe infiltrates. We herein describe a case of pulmonary M. szulgai infection that shows clinical and radiological presentations similar to active pulmonary tuberculosis.
Mycobacterium szulgai (M. szulgai) is a slow-growing non-tuberculous mycobacteria (NTM) that was first described in 1972 by Marks et al. (1). It was named after Dr. T. Szulga, who developed the lipid analysis method that identified this NTM as a new species (2). It is rarely isolated from human specimens, yet it causes disease requiring treatment (3, 4). Pulmonary disease is the most common manifestation of M. szulgai infection, and it may clinically and radiologically resemble pulmonary tuberculosis (5, 6). M. szulgai infection responds well to antimycobacterial combination chemotherapy (3, 6). Only a few reports have been published about pulmonary M. szulgai infection in Korea (7, 8). Here, we report a case of M. szulgai lung disease in Korea with clinical and radiological presentations similar to active pulmonary tuberculosis, and a good response to antimycobacterial combination treatment. Our Institutional Review Board approved this retrospective study with a waiver of informed consent.
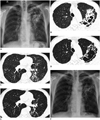
A 55-year-old man visited our hospital for cough and sputum, which had started 2 months before and had gradually aggravated. He experienced a 4 kg weight loss during the past 4 years. He did not have a history of tuberculosis. He was a heavy alcohol user and ex-smoker with a smoking history of 30 pack-years. He lived in a rural area and did not have any special travel history. He worked as a stonemason for 40 years. Chest radiography and routine laboratory tests were performed. An initial chest radiograph disclosed cavitary consolidation in the left upper lung zone (Fig. 1A). Subsequent high-resolution chest CT (HRCT) scans revealed dense peribronchial consolidation with bronchiectatic cavities in the left upper lobe and the superior segment of left lower lobe, and multiple small peribronchial nodular infiltrates in the posterior basal segment of the left lower lobe (Fig. 1B, C). These imaging findings were suggestive of active pulmonary tuberculosis. Acid fast bacilli were observed on a sputum microscopic examination. Therefore, treatment with isoniazid, pyrazinamide, ethambutol and rifampicin was started with suspicion of active pulmonary tuberculosis. His respiratory symptoms and radiological findings improved gradually with antituberculous medication. Two months after the initial treatment, M. szulgai was identified in two separate sputum cultures, and Mycobacterium szulgai (M. szulgai) culture results were negative. The therapeutic regimen was changed to clarithromycin, ethambutol, and rifampicin according to the antibiogram. His symptoms and radiological findings improved further. Chest HRCT scans, performed 4 months after treatment (2 months after changing the therapeutic regimen), showed decreased extent of dense peribronchial consolidation and nodular infiltration in the left lung (Fig. 1D, E). He continued treatment, and the 6 month follow-up chest radiograph shows further improvement of pulmonary infiltrates (Fig. 1F).
Non-tuberculous mycobacteria are referred to as a grouping of all Mycobacterium species other than the obligate pathogens of the M. tuberculosis complex and Mycobacterium leprae (M. leprae). In recent decades, NTM are increasingly recognized as pathogenic to humans. Human diseases due to NTM include pulmonary disease, lymphadenitis, cutaneous disease, and disseminated disease. Among these, pulmonary disease is the most common manifestation. Unlike M. tuberculosis, which is an obligate human pathogen with no environmental reservoir, NTM are commonly isolated from environmental sources such as water and soil. Therefore, respiratory isolates of NTM must be examined to determine whether they are derived from contamination or from colonization with true NTM pulmonary disease (7, 8, 9).
In 2007, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) published diagnostic criteria for NTM infection. The guidelines consist of clinical, radiological, and microbiological criteria as follows; 1) pulmonary symptoms, nodular or cavitary opacities on chest radiograph, or a HRCT scan that show multifocal bronchiectasis with multiple small nodules, 2) appropriate exclusion of other diagnoses, such as tuberculosis, and 3) positive culture results from at least two separate expectorated sputum samples (3). Our case met the ATS/IDSA diagnostic criteria with chronic cough, upper lobe cavitary infiltrates on chest radiograph and HRCT scans, positive culture results from two separate sputum specimens, and negative results for M. tuberculosis. Moreover, M. szulgai is rarely discovered in the environment. Its isolation from respiratory specimens almost always has pathological significance (3, 10).
Non-tuberculous mycobacteria pulmonary disease is radiologically classified into two groups based on HRCT patterns: fibrocavitary disease and nodular bronchiectatic disease. The first type is characterized by cavitary lesions that mostly involve the upper lobes and resemble pulmonary tuberculosis. The second type is characterized by bronchiectasis and nodular lesions, particularly with centrilobular nodules and tree-in-bud appearance, and predominantly distributed in the middle lobe and lingula. The second pattern is the typical appearance of Lady Windermere syndrome (9, 11). The majority of the previously reported M. szulgai pulmonary infections were the fibrocavitary form (4, 8), as shown in this case.
Radiological findings of TB and NTM overlap considerably. Bilateral pulmonary involvement or lower lobe predominance of centrilobular nodules may help differentiate NTM from TB infection. However, upper lobe fibrocavitary lesion as in our case may be indistinguishable from active pulmonary tuberculosis (12).
Clinical characteristics of pulmonary M. szulgai disease are also similar to pulmonary tuberculosis, as shown in this case, with chronic cough, sputum, and weight loss. The majority of patients are men > 50 years with risk factors including alcohol abuse, smoking, chronic obstructive pulmonary disease, and a history of pulmonary TB (4). In contrast to other NTM, M. szulgai is susceptible to most anti-tuberculous drugs. Successful chemotherapy with more than two anti-tuberculous drugs has been reported (3, 4). In addition to traditional agents, M. szulgai is also susceptible to macrolides and fluoroquinolones. Clarithromycin with ethambutol and rifampin or rifabutin was preferred as a therapeutic regimen in some reports (2, 6). At first, our patient was misdiagnosed with active pulmonary tuberculosis due to similar clinical presentations and radiological findings. During the first 2 months until M. szulgai was identified, he felt his symptoms improve under the antituberculous regimens. Thus, it is considered that traditional standard regimens for tuberculosis are also effective for M. szulgai infection to some degree.
In summary, M. szulgai is an unusual pathogen, yet has a pathological significance that causes disease. Pulmonary disease is the most common manifestation of M. szulgai infection, and it may clinically and radiologically resemble pulmonary tuberculosis. It responds well to antimycobacterial combination therapy. Knowledge of the clinical presentation and radiological findings are important as well as bacteriological tests for a correct diagnosis and successful treatment of this rare infection.
Figures and Tables
Fig. 1
55-year-old man with Mycobacterium szulgai pulmonary disease.
A. Initial chest radiograph shows dense peribronchial consolidation containing cavity in left suprahilar area. Note multiple tiny nodules in both lungs. B, C. Lung window images of initial chest high resolution chest computed tomography (HRCT) (1.0-mm section thickness) at levels of aortic arch (B) and bronchus intermedius (C), respectively, show dense peribronchial consolidation containing bronchiectatic cavity in left upper lobe and multiple small nodules and branching linear opacities (tree-in-bud pattern) in left lower lobe. Note multiple small well-defined nodules in right lung with centrilobular and perilymphatic distribution, which are considered to be silicotic nodules. D, E. Lung window images of follow-up chest HRCT obtained 4 months after treatment demonstrate interval decreased extent of peribronchial consolidation and nodular infiltrates in left lung. No significant interval change is noted in extent of silicotic nodules. F. Follow-up chest radiograph 6 months after treatment shows decreased extent of pulmonary infiltrates in left lung.
References
1. Marks J, Jenkins PA, Tsukamura M. Mycobacterium szulgai--a new pathogen. Tubercle. 1972; 53:210–214.
2. van Ingen J, Boeree MJ, de Lange WC, de Haas PE, Dekhuijzen PN, van Soolingen D. Clinical relevance of Mycobacterium szulgai in The Netherlands. Clin Infect Dis. 2008; 46:1200–1205.
3. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
4. Sánchez-Alarcos JM, De Miguel-Díez J, Bonilla I, Sicilia JJ, Alvarez-Sala JL. Pulmonary infection due to Mycobacterium szulgai. Respiration. 2003; 70:533–536.
5. Ohta H, Miyauchi E, Ebina M, Nukiwa T. A case of cutaneous infection caused by mycobacterium szulgai with progression to acute respiratory distress syndrome. Clin Med Insights Case Rep. 2011; 4:29–33.
6. Randhawa K, Smethurst P, Soo Hoo GW. Nine-year progression of untreated pulmonary Mycobacterium szulgai infection. South Med J. 2010; 103:828–830.
7. Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002; 3:145–157.
8. Yoo H, Jeon K, Kim SY, Jeong BH, Park HY, Ki CS, et al. Clinical significance of Mycobacterium szulgai isolates from respiratory specimens. Scand J Infect Dis. 2014; 46:169–174.
9. van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:103–109.
10. Piersimoni C, Scarparo C. Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis. 2008; 8:323–334.
11. Polverosi R, Guarise A, Balestro E, Carloni A, Dalpiaz G, Feragalli B. High-resolution CT of nontuberculous mycobacteria pulmonary infection in immunocompetent, non-HIV-positive patients. Radiol Med. 2010; 115:191–204.
12. Erasmus JJ, McAdams HP, Farrell MA, Patz EF Jr. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics. 1999; 19:1487–1505.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download