Abstract
Massive thoracoabdominal aortic thrombosis is a rare finding in patients with iatrogenic Cushing syndrome in the absence of any coagulation abnormality. It frequently represents an urgent surgical situation. We report the case of an 82-year-old woman with massive aortic thrombosis secondary to iatrogenic Cushing syndrome. A follow-up computed tomography scan showed a decreased amount of thrombus in the aorta after anticoagulation therapy alone.
Massive thoracoabdominal aortic thrombosis is a rare finding. Particularly, the formation of a friable floating thrombus in the proximal aortic arch creates a life-threatening risk of stroke and peripheral embolization (1, 2, 3, 4, 5). Here, we present the case of an 82-year-old woman with massive thoracoabdominal aortic thrombosis secondary to iatrogenic Cushing syndrome. To our knowledge, this is the first reported case in which massive thoracoabdominal aortic thrombosis with a proximal floating end occurred secondary to iatrogenic Cushing syndrome.
An 82-year-old woman was admitted to the emergency department for dyspnea (New York Heart Association Class III). She had experienced generalized weakness over a 3-day period. Her history included rheumatoid arthritis with long-term glucocorticoid treatment (including deflazacort 9 mg) for 20 years. Her vital signs were as follows: blood pressure, 110/70 mm Hg, pulse, 83/min, and respiratory rate, 20/min. Physical examination revealed central obesity, a rather ambiguous buffalo hump, short neck, and moon face. A diagnosis of iatrogenic Cushing syndrome was made based on the clinical presentation, such as the history of steroid administration and cushingoid morphology.
On hospital admission, chest radiography showed cardiomegaly with mediastinal widening. Electrocardiography revealed sinus rhythm, first-degree atrioventricular block, and low voltage. Laboratory tests were normal except for elevated N-terminal B-type natriuretic peptide, creatine kinase MB fraction, troponin T, and D-dimer (5264 pg/mL, 19.73 ng/mL, 0.268 ng/mL, and 1809 ng/mL, respectively). Laboratory tests for thrombophilia (including lupus anticoagulant, anticardiolipin antibodies, and antiphospholipid antibodies) and internal malignancy screens were negative. Two-dimensional transthoracic echocardiography (TTE) demonstrated an anterior wall motion abnormality and prominent epicardial fat. A suprasternal notch view showed large thrombi in the aortic arch with a proximal floating end (Fig. 1A, Supplemented online video clip). Aortic computed tomography (CT) was performed using a 128-slice scanner (Ingenuity CT, Philips Healthcare, Best, The Netherlands). Contrast-enhanced electrocardiography (ECG)-gated CT was performed from the mid-neck to the inguinal level in a craniocaudal direction using the following parameters: a detector collimation of 64 × 0.625 mm, a gantry rotation time of 330 ms, tube voltage of 100 kV, and 200 mAs per rotation. An aortic CT scan showed extensive thrombi from the distal ascending thoracic aorta to the proximal descending thoracic aorta and distal abdominal aorta (Fig. 1B). Thrombi were not detected in arch vessels, including the brachiocephalic trunk, left common carotid, and subclavian arteries. Coronary CT angiography (CCTA) was performed using a 640-slice scanner (Aquilion ONE, Toshiba Medical Systems, Nasu, Japan). Contrast-enhanced ECG-gated coronary CT was performed from 2 cm below the carina to upper abdomen level in a craniocaudal direction using the following parameters: a detector collimation of 320 × 0.5 mm, a gantry rotation time of 350 ms, tube voltage of 100 kV, and 50 mAs per rotation. CCTA demonstrated a suspicious tight stenosis without definite plaque in the mid left anterior descending coronary artery (Fig. 1C).
During 3 weeks of follow-up, the patient's condition was stable and anticoagulation therapy was started. We prescribed hydrocortisone 10 mg once daily after the diagnosis of thrombus due to adrenal insufficiency. Another aortic CT scan and TTE were performed 3 weeks later, showing a slightly decreased amount of thrombus in the thoracic and abdominal aorta (Fig. 1D). A follow-up suprasternal notch TTE view showed that the amount of thrombus in the aortic arch had decreased and the proximal floating end of the thrombus had disappeared (Fig. 1E). We decided to continue with surveillance and medical therapy. The patient remained asymptomatic. Thus, the patient was discharged on the 33rd hospital day.
Aortic thrombosis is an uncommon, often serious, condition that most commonly involves the abdominal aorta. Formation of a friable floating thrombus, particularly in the proximal aortic arch, creates a life-threatening risk of stroke and peripheral embolization (1, 2, 3, 4, 5). Many factors, such as atherosclerosis, dissection, trauma, malignancy, and coagulopathies, have been associated with aortic thrombosis. It has been demonstrated that steroids may cause direct endothelial injury of large arterial vessels (6). Steroids may act directly on the vascular system, inducing intimal thickening in some predisposed persons. Intimal hyperplasia with or without overlying thrombosis has been reported in women who take oral contraceptives or who are pregnant, and in a few men with hepatic illnesses (7, 8). Histologically, these lesions consist of subendothelial fibrosis with endothelial proliferation (6).
Although malignancy and coagulopathies have been associated with aortic thrombosis, in our patient, the results of thrombophilia and internal malignancy screens were normal. However, the patient had been self-medicated with a potent glucocorticoid for 20 years. Our assumption is that a hypercoagulable state resulting from the medication may have played a thrombogenic role in this case. Jilma et al. (9) suggested that a high-dose glucocorticoids might augment hemostasis, which may contribute to unfavorable vascular events by increasing platelet activation and von Willebrand factor-dependent thrombosis.
Treatment of the aortic thrombosis was considered essential because of the threat of serious systemic embolization. However, there is no consensus regarding whether anticoagulation therapy alone or surgical intervention leads to the most favorable result (3). Furthermore, our patient and attendant did not consent to surgical intervention. The general condition of the patient was poor and she was elderly. Thus, we decided on treatment with anticoagulation therapy alone.
Our patient experienced dyspnea as an initial presentation. This condition seemed to be associated with myocardial ischemia resulting from a tight focal stenosis with an atheromatous plaque in the mid left anterior descending coronary artery, demonstrated with multidetector coronary CT angiography. However, we did not decide to conduct coronary angiography or revascularization of the coronary artery because of the massive aortic thrombosis.
Vascular thrombosis occurs in patients with Cushing syndrome and is a relatively well-known complication. However, a huge floating extensive thrombus in the aorta has not been reported before. This is the first case in which the cause of massive thoracoabdominal aortic thrombosis with proximal floating end in a patient with iatrogenic Cushing syndrome was confirmed by suprasternal notch TTE view and a CT scan.
Figures and Tables
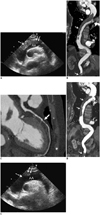 | Fig. 1Massive thoracoabdominal aortic thrombosis in 82-year-old woman with iatrogenic Cushing syndrome.
A. Suprasternal notch view of transthoracic echocardiography showing large thrombi (arrows) in aortic arch (AA) with proximal floating end (dashed arrow). B. Aortic computed tomography (CT) scan with curved multiplanar reformation showing extensive thrombi from distal ascending thoracic aorta to proximal descending thoracic aorta and distal abdominal aorta (arrows). C. Coronary CT angiography with curved multiplanar reformation demonstrating suspicious tight stenosis with no definite plaque in mid left anterior descending coronary artery (arrow). D. Follow-up aortic CT scan with curved multiplanar reformation showing slightly decreased amount of thrombus in thoracic aorta and abdominal aorta (arrows). LV = left ventricle. E. Follow-up suprasternal notch transthoracic echocardiography view showing amount of thrombus in aortic arch (AA) had decreased (arrows) and proximal floating end of thrombus had disappeared (dashed arrow).
|
References
1. Adams C, Nagpal AD, Forbes TL, Lawlor DK, Chu MW. Spontaneous aortic arch thrombus presenting as acute critical limb ischemia. Vasc Endovascular Surg. 2010; 44:309–311.
2. Pousios D, Velissaris T, Duggan S, Tsang G. Floating intra-aortic thrombus presenting as distal arterial embolism. Interact Cardiovasc Thorac Surg. 2009; 9:532–534.
3. Soleimani A, Marzban M, Sahebjam M, Shirani S, Sotoudeh-Anvari M, Abbasi A. Floating thrombus in the aortic arch as an origin of simultaneous peripheral emboli. J Card Surg. 2008; 23:762–764.
4. Mandegar MH, Roshanali F, Kocharian A. Complicated course consequences of a floating thrombus in ascending aorta. Eur J Echocardiogr. 2008; 9:846–848.
5. Hazirolan T, Perler BA, Bluemke DA. Floating thoracic aortic thrombus in "protein S" deficient patient. J Vasc Surg. 2004; 40:381.
6. Nashel DJ. Is atherosclerosis a complication of long-term corticosteroid treatment? Am J Med. 1986; 80:925–929.
7. Irey NS, Norris HJ. Intimal vascular lesions associated with female reproductive steroids. Arch Pathol. 1973; 96:227–234.
8. Lamy AL, Roy PH, Morissette JJ, Cantin R. Intimal hyperplasia and thrombosis of the visceral arteries in a young woman: possible relation with oral contraceptives and smoking. Surgery. 1988; 103:706–710.
9. Jilma B, Cvitko T, Winter-Fabry A, Petroczi K, Quehenberger P, Blann AD. High dose dexamethasone increases circulating P-selectin and von Willebrand factor levels in healthy men. Thromb Haemost. 2005; 94:797–801.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download