Abstract
Objective
The purpose of this study was to evaluate the effectiveness of detachable interlock microcoils for an embolization of the internal iliac artery during an endovascular aneurysm repair (EVAR).
Materials and Methods
A retrospective review was conducted on 40 patients with aortic aneurysms, who had undergone an EVAR between January 2010 and March 2012. Among them, 16 patients were referred for embolization of the internal iliac artery for the prevention of type II endoleaks. Among 16 patients, 13 patients underwent embolization using detachable interlock microcoils during an EVAR. Computed tomographic angiographies and clinical examinations were performed during the follow-up period. Technical success, clinical outcome, and complications were reviewed.
Results
Internal iliac artery embolizations using detachable interlock microcoils were technically successful in all 13 patients, with no occurrence of procedure-related complications. Follow-up imaging was accomplished in the 13 cases. In all cases, type II endoleak was not observed with computed tomographic angiography during the median follow-up of 3 months (range, 1-27 months) and the median clinical follow-up of 12 months (range, 1-27 months). Two of 13 (15%) patients had symptoms of buttock pain, and one patient died due to underlying stomach cancer. No significant clinical symptoms such as bowel ischemia were observed.
Endovascular aneurysm repair (EVAR) is generally a safe and efficient treatment (1, 2, 3). However, as the anatomy of abdominal aortic aneurysm and aneurysmal dilatation of iliac arteries are complex at times, a variety of bifurcated or aorto-uniiliac stent grafts are used to provide an adequate proximal aortic neck length and diameter (1). In the case of short or aneurysmal common iliac arteries, extension of the stent graft into the external iliac artery is required in order to achieve safe positioning and sealing (1, 2). Also, an ipsilateral internal iliac artery embolization is generally necessary to prevent retrograde blood flow and potential endoleaks (1, 2). Internal iliac embolization using conventional pushable coils has been proposed as a standard procedure (1, 4, 5). Although conventional pushable coil embolizations generates thrombosis to provide collateral flow among the distal branches, precise performance of conventional pushable coil embolization of the internal iliac artery may be difficult due to coil migration or placement into more distal internal iliac artery branches (1, 4, 5). An imprecise conventional pushable coil embolization may lead to buttock claudication, sexual dysfunction, and ischemia after occlusion of the internal iliac arteries (4, 5, 6, 7). The Amplatzer vascular plug (AVP) is another embolization device. The AVP provides the advantage of precise positioning. However, the AVP does not have an over-the-wire capability, so a long guiding catheter is needed for advancement; and sometimes, tortuous internal iliac artery could not be selected for AVP embolization (8).
The purpose of this study was to evaluate the effectiveness of a 0.018-inch detachable interlock microcoil (Boston Scientific, Natick, MA, USA) for embolization of the internal iliac artery during an EVAR procedure, in order to overcome the disadvantages associated with the use of a conventional pushable coil embolization.
We received approval of our Institutional Review Board for this retrospective study. A retrospective review was conducted on 40 patients with aortic aneurysm, who had undergone an EVAR between January 2010 and March 2012. Among them, 16 patients were referred for embolization of the internal iliac artery to prevent type II endoleaks. Because all of the patients' aortic aneurysms extended to the common iliac arteries, stent-graft placement across the origin of the internal iliac arteries was needed. Among 16 patients, 12 male patients and one female patient (mean ages, 75 years) underwent embolization using detachable interlock microcoils. Detachable interlock microcoil embolizations were performed to repair nine left internal iliac arteries and four right internal iliac arteries. In 11 patients, the internal iliac arteries showed a medium-sized diameter ranging from 5 mm to 14 mm; and in 2 patients, the internal iliac arteries showed aneurismal dilatation with 6.5 mm and 12 mm in each diameter. The other 3 patients underwent embolization using AVPs for embolization of the internal iliac artery. Due to the cylindrical shape of the AVPs, the specific morphology of the internal iliac arteries with a short portion of constant diameter was suitable for plug embolization. None of the patients underwent additional embolization of other arteries. Aortic aneurysms were classified according to EUROSTAR classification criteria as type A to type E before the procedure (9).
The detachable interlock microcoil is a device composed of a coil and a coil pusher interposed within the catheter by the two interlocking cylinders. The cylinders attached to the coil cannot move beyond the cylinder attached to the coil pusher, until they have been fully discharged (10, 11). The device has two radiopaque markers at the distal end of the microcatheter and one on the distal end of the pusher. The coil attached to the pusher can be advanced until the marker on the distal end of the pusher superposes on the proximal marker of the microcatheter (10, 11). On demand, the coil can be easily recovered before the two markers are superposed and can be pushed forward again. At the point of superposition of the markers, the coil will be discharged with a rotational movement of the pusher (10, 11). The variety of lengths and diameters of usable, detachable interlock microcoils have permitted a made-to-order approach to occluded vessels (11).
Procedures were performed under general anesthesia. Embolizations of internal iliac arteries were performed via a contralateral femoral arterial approach for 10 patients and an ipsilateral femoral arterial approach for 3 patients before the EVAR. A 6-Fr introducer sheath was placed into the right, left, or both femoral arteries. An angiographic pigtail catheter placed above the aortic bifurcation was used to obtain a nonselective diagnostic aortoiliac angiography for identification of the internal iliac artery and measurement of the diameters of the target vessels. Selective catheterization of the targeted internal iliac arteries was then performed. The microcatheter (Ranegade, Boston Scientific, Natick, MA, USA) was advanced until its tip was placed at the target level of the internal iliac artery. After controlling the position of the catheter in the internal iliac artery by injection of a small amount of contrast media, we loaded the coil that comes pre-attached to the pusher in an introducing tube, into a catheter. The detachable interlocking microcoils were placed from the catheter into the internal iliac artery. If the coil was placed distal to the bifurcation of the internal iliac artery and/or was obstructing the first branch vessel of the internal iliac artery, we recovered the coil and pushed forward again. At the point of superpositioning of the markers, the coils were released. The detachable interlocking microcoil embolization was finished with angiographic checking of minimal or absent antegrade flow into the internal iliac artery. Once the embolization procedures had been completed, the EVAR was achieved.
All patients were observed closely after the procedure. After the EVAR, patients were followed up with clinical visits at variable intervals depending on the patient's condition, as is usually done in our vascular surgery department. Follow-up CT angiographies were performed at discharge and at 1, 3, 6, 12, 26, or 27 months with variable frequencies, depending on the patient. Follow-up review of CT angiographies was performed by two interventional radiologists. During the follow-ups, the patients were evaluated for signs and symptoms of buttock claudication, bowel ischemia, and sexual dysfunction.
Our study was conducted in order to evaluate technical success, clinical success, and complication. Technical success was defined as the precise embolization of the neck of the internal iliac artery, not the first branch vessel of the internal iliac artery, and the absence of antegrade flow into the embolized internal iliac artery. Clinical success was defined as the absence of endoleaks caused by retrograde internal iliac artery perfusion and was certificated by follow-up imaging. Evaluation of clinical side effects included symptoms such as buttock claudication, bowel ischemia, and sexual dysfunction. Procedure related complications were also evaluated. Complications of treatment were evaluated on the basis of results, according to the Society of Interventional Radiology clinical practice guidelines (12). Minor complications consisted of outcomes requiring no therapy and having no consequence or formal therapy. Major complications consisted of outcomes requiring short hospitalization period of less than 48 hours, critical therapy, prolonged hospitalization of more than 48 hours, or the outcomes resulting in permanent sequelae or death.
Findings on preprocedural CT angiographies of all patients revealed six cases of type D abdominal aortic aneurysm and seven cases of type E abdominal aortic aneurysm. Internal iliac artery embolization was left-sided in nine patients and right-sided in four patients. All internal iliac artery embolization procedures were performed during the EVAR. The mean number of detachable interlock microcoils used for internal iliac artery embolization was 4.15 (range: 2-9, size: 5-14 mm) (Table 1). Internal iliac artery embolizations were performed successfully in all 13 patients. Intraoperative angiograms confirmed vessel occlusion in all 13 patients, who had undergone perioperative embolization, and accurate embolization of the neck of the internal iliac artery before the first branch vessel in all 13 patients to our original intention. Two examples of embolization in patient 1 and patient 2 are shown in Figures 1, 2. Various lengths and diameters of detachable interlock microcoils were deployed as a tailored approach to the occlusion of the internal iliac artery. The median clinical follow-up period was 12 months (range, 1-27 months). The median imaging follow-up period was 3 months (range, 1-27 months). Postprocedural follow-up imaging was available variably across the patients as follows: at 1 month after the procedure for 3 patients (patient 7, 11, and 12), at 3 months for 4 patients (patient 3, 6, 8, 10), at 6 months for 3 patients (patient 1, 5, 13), at 12 months for 1 patient (patient 9), at 26 months for 1 patient (patient 4), and at 27 months for 1 patient (patient 2). CT angiographies were used for all postoperative imaging. Neither retrograde perfusion of the aneurysm sac nor endoleak related to the detachable interlocking coil was observed on CT angiographies in any patient. Two examples of follow-up imaging of patient 1 and patient 2 are shown in Figures 3, 4.
Clinical side effects attributed to a detachable interlock microcoil embolization were documented in two patients (15%). One patient reported of severe pain in both buttocks (especially the left embolization side) immediately after the embolization. However, this patient reported of continuous improvement of symptoms at three-month follow-up.
Another patient reported of mild right buttock pain immediately after the embolization. However, the pain had nearly disappeared by the three-month follow-up. None of the patients reported of bowel ischemia or sexual dysfunction. One patient died due to underlying stomach cancer during the follow-up period. There were no minor complications such as nausea, vomiting, and groin hematoma or major complication such as coil migration, bleeding, sepsis, or death.
Anatomic feasibility of EVARs has been reported to be between 10% and 80% (13, 14). An aneurysmal dilatation of the iliac artery can prevent safe anchoring of the distal graft. In our trial, all patients with an abdominal aorta aneurysm were selected to receive internal iliac artery embolization on the basis of the special anatomy of the common iliac arteries. All patients had an accompanying aneurysmal common iliac artery with extension into the iliac bifurcation, which did not allow sufficient distal stent-graft sealing. Because the anatomy of these patients required elongation of the stent-graft into the external iliac artery, an internal iliac artery embolization was compulsory to prevent retrograde perfusion and potential endoleaks.
In the past, the standard strategy most oftenly recommended included an internal iliac artery embolization using a conventional pushable coil to prevent endoleaks and potential reflux (1, 4, 5). The effectiveness of conventional pushable coils for the occlusion of an artery has been established beyond any doubt (15). In general, conventional pushable coil embolization of the internal iliac artery is a safe approach that can result in a significant increase in the applicability of an EVAR (4, 5). The initial technical success rate of the conventional pushable coil embolization was about 95% (16). However, precise embolization of the internal iliac artery using the conventional pushable coil may be difficult due to coil migration or placement into more distal internal iliac artery branches. Therefore, inaccurate coil embolization may lead to buttock claudication, sexual dysfunction, and ischemia after occlusion of internal iliac arteries (5, 7). Park et al. (17) reported an internal iliac artery embolization with coils associated EVAR that can lead to ischemic complication such as buttock claudications. Also, a conventional pushable coil cannot be repositioned once it has been discharged into the blood vessels. Therefore, the released coil cannot be retracted when it shows instability in a blood vessel (18). A nontarget coil embolization risk was reported to be 9-23% (19, 20). Ischemic complications after conventional pushable coil embolizations are quite common, and the severity ranges from buttock claudication or erectile dysfunction to colonic perforation or gluteal necrosis. Buttock claudication is the most commonly reported, from 12% to 58%; and the erectile dysfunction has been reported at 0% to 20% (2, 21, 22).
Controlled-release coils such as the detachable interlocking microcoils have been developed in an effort to overcome this major limitation of conventional pushable coil embolization (23). With the detachable interlock microcoils, a mechanical joining is made between the coil and its pusher wire (11). If the junction of the distal end of the pusher wire and the proximal end of the coil interlock is within the microcatheter, the coil can be freely advanced or recovered; only when it is beyond the tip of the microcatheter dose the coil mechanically detaches. Just after a detachable interlock microcoil has been released from the tip of the catheter, the coil has detached itself. This mechanism supports the placement of detachable interlock microcoils at a desired location before being totally discharged (11). As with this mechanism, we successfully embolized the internal iliac artery avoiding coil migrations. Although the number of patients receiving the conventional pushable coil embolization in our hospital was small, the ratio of postembolization ischemic complications (50%) was greater than that of the patients receiving the detachable interlock microcoil (15%). These complications were improved during the follow-up period. Reidy and Qureshi (11) reported on the use of detachable interlock microcoils in situations where the use of conventional pushable coil embolization techniques was considered unsafe. In this study, control of the coil and its discharge were acceptable, and all coils were fully retrievable up to the point of deployment (11). From these results, the investigators inferred that the coil is an effective device that allows the performance of controlled embolization (11). Also, Dudeck et al. (24) compared embolization of the gastroduodenal artery before selective internal radiotherapy for alleviation in primary and secondary liver tumors using the conventional pushable coil with the detachable interlock microcoil. The detachable interlock microcoil was associated with a shorter procedure time, lower radiation dose for coil deployment, and lower coil number than the conventional pushable coil (24). Also, endovascular detachable coil embolization could be an effective treatment for renal artery aneurysms (25). Even difficult renal artery aneurysm cases such as a wide-necked or bifurcations could be successfully treated by the detachable interlock coil (25).
Some investigators have reported success with the use of AVPs in internal iliac artery embolizations before endovascular repair of aortoiliac aneurysms (2, 8). Precise positioning and cost saving are the advantages of the AVP, compared to the coils. There are no reports of initial technical failure of AVPs embolization in the literature (2, 8, 26). However, there are some reported cases of the need for additional coils or a secondary device deployment (16). Also, the AVP does not have an over the wire capability, therefore, a long guiding catheter is needed for the forward motion of the AVPs. As a consequence, progression of the AVPs through the long guiding catheter may be blocked, and sometimes, tortuous internal iliac artery could not be selected for AVP embolization (8). Meanwhile, recently launched AVP 4 (AGA Medical Corp., Plymouth, MN, USA) is accessible with the diameters of 4-8 mm. The advantages over the previous vascular plug is that it may be easier to move through tortuous vasculature and there may be no need for sheath or guiding catheter exchange (27). However, the AVP 4 can be used to embolize the small internal iliac arteries of less than 6.5 mm in diameter. In our study, most cases (12 cases, 92%) had diameters greater than 6.5 mm, so these were not suitable for the AVP 4. The AVP 4 was launched in Korea in late 2012, but our procedures were done between 2010 and 2012; so, we were unable to use the AVP 4. Buttock claudication rates of AVPs embolization are reported in 9% to 33% of the cases, and erectile dysfunctions were essentially unreported (2, 8, 16, 26). Libicher et al. (28) reported that embolization of internal iliac arteries with either coils or AVPs was safe and effective. Initial buttock claudication was more severe with coils, but there was no significant difference after 12 months of follow-up (28). AVPs embolization is connected with a significant reduction of procedure time and radiation dose compared to coils (28).
In our detachable interlock microcoil study, buttock pain occurred in two patients (15%). Buttock claudication is the most common ischemic complication after interruption of the internal iliac artery. Schoder et al. (1) reported a greater ratio of postembolization buttock claudications when coils were placed in (47%) versus above (20%) of internal iliac artery bifurcation. Also in ischemic complications of our conventional pushable coil embolizations, the coils were located inaccurately in the internal artery bifurcation. Some reported that the majority of milder buttock claudication improved over time with conservative treatment such as that in our patients (29, 30, 31).
We embolized the internal iliac artery before the first branch. Therefore, the ratio of postembolization buttock pain was small and the patients' symptoms showed improvement at the three-month follow-up. Therefore, precise positioning of the coils was critical in preventing buttock caludication or other complications. In most patients, the intricate network of collateral vessels of the pelvis compensated for ischemic complications of embolization of the internal iliac arteries (29).
This study had limitations. First, the sample size is small, which prevents us from making generalizations on the basis of the results of this study. Second, this series is consecutive and lacks randomization; therefore, patient selection bias may have played a role. A prospective randomized trial would be beneficial in order to define the exact value and clinical outcome of internal iliac artery embolization using detachable interlock microcoils compared to conventional coils. Third, the follow-up interval was short and uneven.
In conclusion, internal iliac artery embolization during an EVAR using detachable interlock microcoils to prevent type II endoleaks appears safe and effective, although this should be further proven in a larger population.
Figures and Tables
Fig. 1
63-year-old man (patient 1) with type E abdominal aortic aneurysm had left IIA embolization before EVAR.
A. Preprocedural digital subtraction image demonstrates type E AAA. B. Preprocedural digital subtraction image documents placement of sheath within left IIA. C. Digital subtraction image shows correct positioning of detachable interlock microcoil within left IIA. D. Digital subtraction image after procedure documents successful exclusion of left IIA. AAA = abdominal aortic aneurysm, EVAR = endovascular aneurysm repair, IIA = internal iliac artery
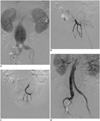
Fig. 2
78-year-old man (patient 2) who has AAA with short and dilated left CIA had left IIA embolization before EVAR.
A. Preprocedural digital subtraction image demonstrates type E AAA. B. Preprocedural digital subtraction image documents placement of sheath within left IIA. C. Digital subtraction image shows correct positioning of detachable interlock microcoil within left IIA. D. Digital subtraction image after procedure documents successful exclusion of left IIA. AAA = abdominal aortic aneurysm, CIA = common iliac artery, EVAR = endovascular aneurysm repair, IIA = internal iliac artery
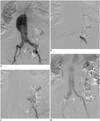
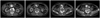
Fig. 3
63-year-old man (patient 1) with type E abdominal aortic aneurysm had left IIA embolization before EVAR. After 1 month, postprocedural CT angiography image did not document endoleaks related to detachable interlocking coil. EVAR = endovascular aneurysm repair, IIA = internal iliac artery

Fig. 4
78-year-old man (patient 2) who had AAA with short and dilated left CIA had left IIA embolization before EVAR. After 1 month, postprocedural CT angiography image did not document endoleaks related to detachable interlocking coil. AAA = abdominal aortic aneurysm, CIA = common iliac artery, EVAR = endovascular aneurysm repair, IIA = internal iliac artery
References
1. Schoder M, Zaunbauer L, Hölzenbein T, Fleischmann D, Cejna M, Kretschmer G, et al. Internal iliac artery embolization before endovascular repair of abdominal aortic aneurysms: frequency, efficacy, and clinical results. AJR Am J Roentgenol. 2001; 177:599–605.
2. Ha CD, Calcagno D. Amplatzer Vascular Plug to occlude the internal iliac arteries in patients undergoing aortoiliac aneurysm repair. J Vasc Surg. 2005; 42:1058–1062.
3. Murphy KD, Richter GM, Henry M, Encarnacion CE, Le VA, Palmaz JC. Aortoiliac aneurysms: management with endovascular stent-graft placement. Radiology. 1996; 198:473–480.
4. Lee C, Dougherty M, Calligaro K. Concomitant unilateral internal iliac artery embolization and endovascular infrarenal aortic aneurysm repair. J Vasc Surg. 2006; 43:903–907.
5. Cynamon J, Lerer D, Veith FJ, Taragin BH, Wahl SI, Lautin JL, et al. Hypogastric artery coil embolization prior to endoluminal repair of aneurysms and fistulas: buttock claudication, a recognized but possibly preventable complication. J Vasc Interv Radiol. 2000; 11:573–577.
6. Marin ML, Veith FJ, Lyon RT, Cynamon J, Sanchez LA. Transfemoral endovascular repair of iliac artery aneurysms. Am J Surg. 1995; 170:179–182.
7. Razavi MK, DeGroot M, Olcott C 3rd, Sze D, Kee S, Semba CP, et al. Internal iliac artery embolization in the stent-graft treatment of aortoiliac aneurysms: analysis of outcomes and complications. J Vasc Interv Radiol. 2000; 11:561–566.
8. Kickuth R, Dick F, Triller J, Ludwig K, Schmidli J, Do DD. Internal iliac artery embolization before endovascular repair of aortoiliac aneurysms with a nitinol vascular occlusion plug. J Vasc Interv Radiol. 2007; 18:1081–1087.
9. Harris PL, Buth J, Mialhe C, Myhre HO, Norgren L. EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair. The need for clinical trials of endovascular abdominal aortic aneurysm stent-graft repair: The EUROSTAR Project. J Endovasc Surg. 1997; 4:72–77. discussion 78-79.
10. Cekirge HS, Saatci I, Firat MM, Balkanci F, Besim A. Interlocking detachable coil occlusion in the endovascular treatment of intracranial aneurysms: preliminary results. AJNR Am J Neuroradiol. 1996; 17:1651–1657.
11. Reidy JF, Qureshi SA. Interlocking detachable platinum coils, a controlled embolization device: early clinical experience. Cardiovasc Intervent Radiol. 1996; 19:85–90.
12. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S199–S202.
13. Armon MP, Yusuf SW, Latief K, Whitaker SC, Gregson RH, Wenham PW, et al. Anatomical suitability of abdominal aortic aneurysms for endovascular repair. Br J Surg. 1997; 84:178–180.
14. Brewster DC, Geller SC, Kaufman JA, Cambria RP, Gertler JP, LaMuraglia GM, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcome of conventional open repair. J Vasc Surg. 1998; 27:992–1003. discussion 1004-1005.
15. Young AT, Tadavarthy SM, Yedlicka JW Jr, Hunter DW, Becker GJ, Herrera MA, et al. Vasculary embolotherapy. In : Castañeda-Zúñiga WR, Tadavarthy SM, editors. Interventional radiology. 2nd ed. Baltimore: Williams & Wilkins;1992. p. 9–200.
16. Vandy F, Criado E, Upchurch GR Jr, Williams DM, Rectenwald J, Eliason J. Transluminal hypogastric artery occlusion with an Amplatzer vascular plug during endovascular aortic aneurysm repair. J Vasc Surg. 2008; 48:1121–1124.
17. Park KM, Yang SS, Kim YW, Park KB, Park HS, Do YS, et al. Clinical outcomes after internal iliac artery embolization prior to endovascular aortic aneurysm repair. Surg Today. 2014; 44:472–477.
18. Nancarrow PA, Fellows KE, Lock JE. Stability of coil emboli: an in vitro study. Cardiovasc Intervent Radiol. 1987; 10:226–229.
19. Krüger K, Wagner D, Gawenda M, Strohe D, Uedelhoven J, Brunkwall J, et al. [Coil embolization of arteriovenous fistulae on in situ saphenous vein bypasses: success rate and complications]. Rofo. 2007; 179:587–592.
20. Goh RH, Sniderman KW, Kalman PG. Long-term follow-up of management of failing in situ saphenous vein bypass grafts using endovascular intervention techniques. J Vasc Interv Radiol. 2000; 11:705–712.
21. Farahmand P, Becquemin JP, Desgranges P, Allaire E, Marzelle J, Roudot-Thoraval F. Is hypogastric artery embolization during endovascular aortoiliac aneurysm repair (EVAR) innocuous and useful? Eur J Vasc Endovasc Surg. 2008; 35:429–435.
22. Mehta M, Veith FJ, Ohki T, Cynamon J, Goldstein K, Suggs WD, et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg. 2001; 33:2 Suppl. S27–S32.
23. Marks MP, Chee H, Liddell RP, Steinberg GK, Panahian N, Lane B. A mechanically detachable coil for the treatment of aneurysms and occlusion of blood vessels. AJNR Am J Neuroradiol. 1994; 15:821–827.
24. Dudeck O, Bulla K, Wieners G, Ruehl R, Ulrich G, Amthauer H, et al. Embolization of the gastroduodenal artery before selective internal radiotherapy: a prospectively randomized trial comparing standard pushable coils with fibered interlock detachable coils. Cardiovasc Intervent Radiol. 2011; 34:74–80.
25. Seo JM, Park KB, Kim KH, Jeon P, Shin SW, Park HS, et al. Clinical and multidetector CT follow-up results of renal artery aneurysms treated by detachable coil embolization using 3D rotational angiography. Acta Radiol. 2011; 52:854–859.
26. Pellerin O, Caruba T, Kandounakis Y, Novelli L, Pineau J, Prognon P, et al. Embolization of the internal iliac artery: cost-effectiveness of two different techniques. Cardiovasc Intervent Radiol. 2008; 31:1088–1093.
27. Ferro C, Rossi UG, Bovio G, Petrocelli F, Seitun S. The Amplatzer vascular plug 4: preliminary experience. Cardiovasc Intervent Radiol. 2010; 33:844–848.
28. Libicher M, Pavlidis D, Bangard C, Gawenda M. Occlusion of the internal iliac artery prior EVAR: comparison of coils and plugs. Vasc Endovascular Surg. 2012; 46:34–39.
29. Yano OJ, Morrissey N, Eisen L, Faries PL, Soundararajan K, Wan S, et al. Intentional internal iliac artery occlusion to facilitate endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2001; 34:204–211.
30. Mehta M, Veith FJ, Ohki T. When should the internal iliac arteries be preserved in patients undergoing endovascular or open repair of aortoiliac aneurysms? Vasc Surg Endovasc Ther. 2001; 14:17–28.
31. Karch LA, Hodgson KJ, Mattos MA, Bohannon WT, Ramsey DE, McLafferty RB. Adverse consequences of internal iliac artery occlusion during endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2000; 32:676–683.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download