Abstract
Cowden syndrome is an uncommon, autosomal dominant disease which is characterized by multiple hamartomas of the skin, mucous membrane, brain, breast, thyroid, and gastrointestinal tract. The diagnosis of Cowden syndrome implicates an increased risk of developing breast cancer. We report a case of a 22-year-old woman with Cowden syndrome that presented as breast cancer with concomitant bilateral exuberant benign masses in both breasts.
Cowden syndrome is an uncommon, autosomal dominant disease characterized by multiple hamartomas of the skin, mucous membrane, brain, breast, thyroid, and gastrointestinal tract (1). Its most relevant clinical feature is an increased risk of developing breast cancer. This risk is reported to be 20-50% in patients with Cowden disease compared to 12% in the general women population (1, 2, 3). So, the National Comprehensive Cancer Network (NCCN) recommends an annual screening with breast MRI and mammography in women with Cowden syndrome starting between 30 and 35 years of age (4). Herein we report a case of Cowden syndrome in a young woman with phosphatase and tensin homolog (PTEN) gene mutation. The syndrome presented as breast cancer with exuberant benign masses in both breasts, thyroid cancer, macrocephaly, intraoral papules, and an arteriovenous malformation (AVM) of the parotid gland. This case led to a retrospective review of imaging and clinical findings. The review was approved by the Institutional Review Board of our institution. An informed consent was waived.
A 19-year-old woman presented with a lump in her right breast. She had macrocephaly and milimetric papules on the tongue giving a cobble stone appearance. The breast ultrasonography (US) showed more than twenty circumscribed, oval masses in each breast. An US-guided core biopsy was performed for the palpable masses which were confirmed as juvenile fibroadenomas. After the biopsy, a breast US was done as follow-up every 6 or 12 months for 3 years. During the surveillance period, core biopsies were performed six times, followed by US-guided vacuum-assisted core needle excisions or surgical excisions of lesions with increased sizes. Pathologic results were fibroadenoma, tubular adenoma or atypical ductal hyperplasia (ADH) involving fibroadenoma.
At the age of 19 years, the patient underwent a neck US for a palpable mass in the left neck. Several indeterminate nodules were revealed in both thyroid glands. A follow-up US revealed that some of those nodules increased in size. The patient underwent a total thyroidectomy and the pathologic result revealed an invasive follicular carcinoma in the left thyroid and follicular adenoma in the right thyroid (Fig. 1A). At the age of 21 years, she visited the hospital due to a swelling of her left cheek. Head and neck CT scan revealed a vascular mass in the left parotid gland extended into the left forehead. An external carotid angiography confirmed the diagnosis of an AVM with feeder vessel arising from the left internal maxillary artery (Fig. 1B). A partial embolization decreased the blood flow through the AVM, alleviating the patient's symptom. She was referred to genetic counseling under the suggestion of a Cowden syndrome and the PTEN DNA sequencing test of her blood sample revealed a frameshift mutation, c.301dupA (p.I101NfsX6).
At the age of 22 years, the patient presented with a mass with increased size and increased vascularity in the left breast. An US-guided vacuum-assisted core needle excision revealed a ductal carcinoma in situ (DCIS) of non-comedo type and a low grade involving fibroadenoma (Fig. 1C). The contrast-enhanced breast MRI for the preoperative evaluation demonstrated multiple, well-circumscribed, enhancing masses in both breasts (Fig. 1D). All masses showed high or intermediate signal intensity on T2-weighted images (Fig. 1E). Several masses showed an early rapid enhancement with washout kinetic pattern on time-signal intensity curve evaluation, which tends to be associated with malignancy (Fig. 1F). A bilateral prophylactic mastectomy with immediate breast reconstruction was performed in view of multiple bilateral breast masses with suspicious kinetic features on breast MRI and a high risk for breast cancer of Cowden syndrome. The surgical histopathology revealed ADH involving tubular adenoma without residual carcinoma in the left breast and multiple tubular adenomas, fibroadenomas and intraductal papillomas in both breasts. The patient had no family history of breast cancer or Cowden syndrome.
Cowden syndrome is a rare condition characterized by the presence of multiple hamartomas of the skin, mucous membrane, brain, breast, thyroid, and gastrointestinal tract. It has been reported to be susceptible to breast cancer, thyroid cancer, endometrium cancer, renal cell cancer, colorectal cancer, and melanoma (3, 4, 5, 6, 7). The condition was first described in 1963 and was named following the patient's name (8). Current estimates suggest the incidence of Cowden syndrome to be approximately 1 of 200000 (6). The diagnosis is based on pathognomonic clinical features, however, the Cowden syndrome is manifested with highly variable symptoms and signs and might be underdiagnosed. Notably, the characteristic mucocutaneous hamartomatous lesions are generally present prior to the development of internal malignancies (5).
The Cowden syndrome is an autosomal dominant disorder that has been linked to germline mutations in the PTEN gene located on chromosome 10q23. Approximately 85% of patients with Cowden syndrome have an identifiable mutation in their PTEN gene. In our patient, the diagnosis of Cowden syndrome was further supported by the presence of a frameshift mutation of the PTEN gene. Clinical features of our patient constitute one pathognomic criterion (intraoral milimetric papules), three major criteria (breast cancer, thyroid cancer, and macrocephaly), and two minor criteria (fibrocystic breast disease and thyroid adenoma). Those features are sufficient for the diagnosis of Cowden syndrome according to its diagnostic criteria (9). Two pathognomic criteria for Cowden syndrome are: Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma) and mucocutaneous lesions. Major criteria are breast cancer, thyroid cancer (especially follicular), macrocephaly and endometrial cancer. Minor criteria are other thyroid lesions, mental retardation, gastrointestinal hamartomas, fibrocystic breast disease, lipomas, fibromas, genitourinary tumors, genitourinary structural malformations and uterine fibroids.
Nearly all reported women with Cowden syndrome have an involvement of the breast, including both benign and malignant conditions. So, Cowden syndrome was included as a high risk factor for breast cancers and an annual screening MRI was indicated therefore (2, 4, 7). Breast cancer was also reported as the most common malignancy involving women with Cowden syndrome (7). Similar to breast cancers in breast cancer antigen (BRCA) gene mutation carrier women, breast cancers in Cowden syndrome tend to occur in young patients (commonly found between 38 and 46 years of age) than sporadic cancer. In addition, bilateral cancers are reported in 25% of the patients (6). An infiltrating ductal carcinoma or DCIS counts for the majority of breast cancers in patients with Cowden syndrome (1). Nipple or areolar malformations, fibroadenomas, proliferative fibrocystic diseases, ADH or lobular carcinoma in situ have been described for the benign breast disease (1). Multiple tubular adenomas or bilateral exuberant hamartomas and fibroadenomas were reported in patients with Cowden syndrome (5, 10), similar to our case. Therefore, the presence of bilateral extensive breast lesions in very young women should elicit the possibility of an underlying genetic disease that is associated with an increased risk of breast cancer such as the Cowden syndrome.
Thyroid cancer is the second most common associated cancer in patients with Cowden syndrome and the risk of developing thyroid cancer is reported to be 3-10%, compared to less than 1% in the general population (1). The thyroid malignancy in Cowden syndrome exclusively appears as papillary or follicular cancer. A childhood onset has been reported although the average age of thyroid cancer patients with Cowden syndrome has not been reported yet (11). AVMs are not widely recognized features in a Cowden syndrome, but there have been some reports of visceral AVMs (12). Turnbull et al. (12) suggested that the association of AVM with the mutation of PTEN gene is related with the role of PTEN gene in the regulation of the angiogenesis. Owing to the regional symptoms and the possible risk of hemodynamic problems in AVMs, it is important to recognize AVM as a clinical feature of the Cowden syndrome.
With regard to the surveillance guidelines for breast cancer, the NCCN recommends breast self-examination beginning at the age of 18 years, annual clinical breast examination at 25 and mammography and breast MRI at 30 to 35 years of age (or 5 to 10 years before a family's earliest known breast cancer diagnosis) (4). In addition, Bubien et al. (7) suggested a new surveillance recommendation as follows: if severe breast dystrophy exists, annual clinical examination and breast MRI beginning at 20 years of age is recommended and a prophylactic mastectomy can be discussed starting at the age of 25 to 30 years. The NCCN management guidelines propose to start thyroid US at the age of 18 years and the consideration of an annual dermatologic examination and blind endometrial suction biopsy starting at the age of 35 years (4). Acknowledging this will allow the radiologist to recommend the appropriate surveillance for patients with Cowden syndrome.
In conclusion, we have reviewed and discussed the case of a young woman with Cowden syndrome, presented as breast cancer, thyroid cancer, macrocephaly, intraoral papules and AVM of the parotid gland. The awareness of a Cowden syndrome can be helpful for an early diagnosis of related malignancies (especially in breast and thyroid) as well as a forewarning of problems with hamartomas or AVMs.
Figures and Tables
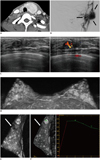 | Fig. 1Left breast cancer, multiple bilateral benign breast masses, left thyroid cancer and arteriovenous malformation (AVM) in left parotid gland in 22-year-old woman with Cowden syndrome.
A. Post-contrast neck CT scan shows tumor in left thyroid gland which was revealed as invasive follicular carcinoma at surgery. B. External carotid angiography confirmed diagnosis of AVM with feeder vessel arising from left internal maxillary artery (arrows). C. Breast ultrasonography (US) shows oval, circumscribed, hypoechoic mass. Color Doppler US of mass shows increased vascularity in center of mass. US-guided vacuum assisted core needle excision for lesion revealed ductal carcinoma in situ involving fibroadenoma. D. Three-dimensional maximum intensity projection image of post-contrast magnetic resonance image after US-guided vacuum assisted excision shows multiple bilateral enhancing masses. E. T2-weighted image shows multiple masses with internal high or intermediate signal intensities and no residual mass in previously excised site (arrow) in left breast. F. Post-contrast T1-weighted sagittal image also shows multiple enhancing masses and no residual mass in previously excised site (arrow) in left breast. Kinetic curve analysis in largest mass shows initial rapid enhancement and delayed washout pattern on dynamic contrast enhanced MR images, suggesting suspicious feature.
|
References
1. Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns. 2009; 18:13–27.
2. Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012; 18:400–407.
3. Jung MH. Breast cancer in Cowden cyndrome: a case report. J Korean Soc Radiol. 2009; 60:279–282.
4. Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD, Friedman S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010; 8:562–594.
5. Sabaté JM, Gómez A, Torrubia S, Blancas C, Sánchez G, Alonso MC, et al. Evaluation of breast involvement in relation to Cowden syndrome: a radiological and clinicopathological study of patients with PTEN germ-line mutations. Eur Radiol. 2006; 16:702–706.
6. Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009; 11:687–694.
7. Bubien V, Bonnet F, Brouste V, Hoppe S, Barouk-Simonet E, David A, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet. 2013; 50:255–263.
8. Lloyd KM 2nd, Dennis M. Cowden's disease. A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963; 58:136–142.
9. Eng C. PTEN hamartoma syndrome. GeneReview at GeneTests: Medical Genetics Information Resource. Updated January 23, 2014. Accessed March 19, 2014. http://www.genetests.org.
10. Melbārde-Gorkuša I, Irmejs A, Bērziņa D, Strumfa I, Aboliņš A, Gardovskis A, et al. Challenges in the management of a patient with Cowden syndrome: case report and literature review. Hered Cancer Clin Pract. 2012; 10:5.
11. Tan WH, Baris HN, Burrows PE, Robson CD, Alomari AI, Mulliken JB, et al. The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. J Med Genet. 2007; 44:594–602.
12. Turnbull MM, Humeniuk V, Stein B, Suthers GK. Arteriovenous malformations in Cowden syndrome. J Med Genet. 2005; 42:e50.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download