INTRODUCTION
Prostate cancer can be deemed as an inevitable geriatric affliction. The prevalence of asymptomatic prostate cancer in the population increases with age. As much as 64% of men between the age of 60-70 years harbor cancerous cells in the prostate (1). Like all cancers, prostate cancer is best managed when diagnosed early as both the recurrence-free survival and the cancer-specific survival are inversely related to the stage of disease at detection (2). Early detection of low-volume prostate cancer relies on the combination of serum prostate-specific antigen (PSA) level and systematic transrectal ultrasound (TRUS)-guided prostate biopsy. TRUS-guided prostate biopsy is the gold standard for diagnosis of prostate cancer (2). It is indicated in all the patients with a raised PSA level or who have an abnormal prostate on digital rectal examination. It is now the most commonly performed invasive procedure in men in a urology setup. However, it is a painful procedure since multiple punctures are made with thick (16-18 gauge) biopsy needles to obtain adequate samples of the prostate. The pain associated with the procedure remains largely ignored in clinical practice. The number of cores taken during prostate biopsy have gradually increased to reduce the sampling error and to improve the cancer detection rate. The current practice is of using an extended biopsy protocol that requires at least 10-12 cores. Incorporating a painful diagnostic procedure as a routine practice is not only unwarranted but can also be considered unethical, particularly when it is performed in a predominantly older age group of patients harboring a low-grade, clinically insignificant disease that does not need aggressive management or treatment at all. Furthermore, the trend is towards increasing the number of cores. Of all the methods that have been evaluated, the periprostatic nerve block is a simple and cost effective method to control pain during TRUS-guided prostate biopsy (3).
Prostate Analgesia: A Historical Perspective
Before the advent of ultrasound, limited samples of a palpable prostate nodule detected on digital rectal examination were obtained directly. Initially, the medical ultrasound was used to assist prostate biopsy via the percutaneous transperineal approach that was technically difficult, time-consuming, and painful. The introduction of TRUS by Takahashi and Ouchi (1963) and the standardization of transrectal biopsy technique by Torp-Pedersen et al. (4) revolutionized prostate biopsy as this approach caused less morbidity. Despite the initial enthusiasm, the TRUS has poor sensitivity in detecting early prostate cancers, which can be as low as 60%. Most early prostate cancers are either occult on sonography or indistinguishable from ubiquitous benign changes in the prostate in the older population (5). A sonographically normal prostate commonly harbors cancer foci. To reduce the sampling error, the biopsy practice evolved from a limited lesion-targeted biopsy to a systematic zone-based sampling of the entire prostate, irrespective of the ultrasound appearance, thereby reducing the role of TRUS to mere biopsy guidance rather than cancer detection per se.
First introduced by Hodge et al. (6) in 1989, the original six core (sextant) biopsy was the standard biopsy practice for prostate cancer diagnosis until studies showed that about 22-30% of cancer can still be missed by a sextant biopsy (7, 8, 9) and that increasing the number of cores improves the cancer detection rate (10, 11). Gradually, the number of cores to be obtained increased to eight (octant biopsy) (12), ten (decant biopsy), and then to the current practice of at least twelve cores. However, the number of cores required for early prostate cancer detection is currently unknown. More cores are needed in an enlarged prostate due to a larger tissue volume (13, 14, 15, 16). More cores are usually required for a repeat biopsy in patients with a persistently elevated PSA level after an initial negative prostate biopsy, sometimes 35 to 60, that are best obtained using stereotactic template mapping through a transperineal approach (saturation biopsies) (17).
Despite an increase in the number of cores and the concomitant pain, the urologists and radiologists did not adopt active pain relief interventions during TRUS-guided prostate biopsy, probably because the rectum was considered to be an insensate structure. The early attempts to mitigate pain evaluated several methods that included general anesthesia, intrarectal local anaesthetic application (18), pudendal and caudal nerve blocks, rofecoxib (19), intravenous propofol (20), nitrous oxide inhalation (21), intravenous conscious sedation (fentanyl and midazolam) (22), intrarectal glyceryl trinitrate (23, 24), intrarectal diclofenac (25), and 40% dimethyl sulfoxide. Some of these methods were of doubtful efficacy (26), while for some methods like intravenous propofol and general anesthesia, a trained team and operating theater-like set-up were needed. Moreover, these methods are time-consuming and impractical. Nash et al. (27) introduced periprostatic nerve block in 1996 when they reported marked pain relief after injection of local anesthetic agent around the prostate.
Pain during TRUS-Guided Prostate Biopsy and the Current Prevalence of Pain Control Measures
Majority of patients perceive TRUS-guided prostate biopsy as a physically and psychologically traumatic experience. The anecdotal clinical experience shows that it is significantly painful in most people and in some cases the patient refuses to undergo the procedure all together (28)
The pain during prostatic biopsy is cumulative in nature (29, 30) i.e., increase in the number of cores is associated with an increase in the total pain (27). Early studies conducted on sextant biopsy without anesthesia showed that 65% to 90% of patients reported discomfort and 30% reported significant pain (31, 32). The mean pain score was 3-4 on the visual analog scale of one to ten (33). Approximately 16% of patients scored greater than or equal to five. Approximately 19% of patients stated that they will not undergo the repeat procedure (34). Six percent of patients judged that the procedure should have been done under general anesthesia (28, 32, 34, 35). In studies of the 8 core (octant) biopsy, approximately 94% of patients found the procedure painful (36) and 24% of patients graded the pain as moderate to severe (28). The extended 12-core prostate biopsy often resulted in excruciating pain, that led to inadequate sampling and abandonment of the procedure before completion (28, 35). Most patients refused when asked if they would agree to undergo the repeat biopsy procedure (34).
A painful experience during prostate biopsy has an important bearing on the patient's well-being and compliance and reduces procedure acceptability. Pain is a subjective sensation and therefore difficult to quantify. Perception and endurance of pain differs from patient to patient with marked inter-subject variations. Pain associated with prostate biopsy is a complex phenomenon with both psychosocial and physical attributes. Psychosocial factors include innate pain threshold of an individual, pre-procedure anxiety, fear of potential cancer diagnosis, social inhibition towards rectal examination, and anxiety about sexual implications. Similarly, physical factors like the patient's age, prostate size, PSA level, pre-procedure rectal enema, history of previous biopsy, initial pain during probe insertion, the portion of the prostate biopsied (35), and the number of cores obtained (37, 38) have all been found to influence pain associated with prostate biopsy.
Pre-procedure anxiety is seen in 67% of patients resulting in exaggerated pain perception (36, 37, 39). Pain during probe insertion has a positive correlation with pain during the subsequent biopsy (40), which is probably related to the anal sphincter tone. Careful counseling prior to the procedure alleviates patient's heightened anxiety and therefore the pain (32).
Younger patients are more susceptible to pain due to low anorectal compliance as a result of higher sphincter tone and due to higher pretest anxiety compared to that in the elderly patients (41, 42). Conversely, older patients have a low pain score during biopsy due to a hypotonic anal sphincter (40).
Pre-procedure enema is reported to reduce pain (38). Apex of the prostate is the most painful site during biopsy (35) due to a predominantly somatic nerve supply to the anorectal mucosa below the dentate line. The repeat prostate biopsy is more painful due to the prior painful experience. It is difficult to achieve pain relief in an enlarged prostate with the periprostatic nerve block and more local anesthetic injections are required (43).
The pre-procedural assessment is essential for identifying the sub-group of patients with risk factors for exaggerated pain and who would require active pain management (35), particularly when pain control measures are used selectively. A typical patient profile with high risk of pain due to prostate biopsy and who will benefit from periprostatic nerve block is a young, anxious man with an enlarged prostate planned for an extended biopsy scheme or who is undergoing a repeat biopsy procedure (35, 43).
The TRUS-guided prostate biopsy is performed by both the urologist and the radiologist. The prostate and the rectum have been considered to be insensate structures and the discomfort associated with the prostate biopsy received little emphasis (3, 28, 44). The pain control methods are underutilized during prostate biopsy, with the reported rate being around ten percent (45, 46). Commonly, the procedure is performed with no or inadequate analgesia (28). A survey revealed that 33% of North American urologists performed the procedure without any form of analgesia (43, 46). Most of them used only the topical anesthetic rectal gel or oral analgesics and a very few of them used periprostatic nerve block for achieving pain control. Their reluctance to incorporate pain controlling measures has resulted in a painful biopsy.
Origin of Pain Associated with Prostate Biopsy and Anatomical Considerations
Pain during prostate biopsy can be due to the following factors:
1) Ultrasound transducer (probe) insertion: the first painful step during TRUS-guided prostate biopsy is the initial ultrasound probe insertion, and it results from mechanical stretching of an un-relaxed anal sphincter and direct contact of the ultrasound transducer (with irregular biopsy-guide attachment). Increased anal tone in a young patient and local inflammatory conditions like fissures or fistula-in-ano exacerbate pain during insertion.
2) Biopsy of the prostate: the most painful step is 10-12 passes of a 16-G or 18-G core-biopsy needle into the prostate and anorectum.
3) Needle punctures while depositing the local anesthetic (in cases with periprostatic nerve block). When the periprostatic nerve block is used, the actual biopsy itself is rendered painless by the block and the few extra needle punctures for periprostatic nerve block add to the total procedural pain and paradoxically, this becomes the most painful step (47, 48).
Anatomical structures that contribute to the pain are as follows:
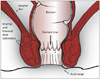
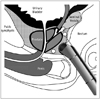
1) Anorectum: from the perspective of a periprostatic nerve block, the anal canal and the rectum can be considered as a single composite structure i.e., the anorectum. Embryonically, the anorectum develops from fusion of the caudal proctodeum and cranial post-allantoic gut. Therefore, the anorectum is richly innervated by both somatic and splanchnic sensory nerves. The watershed between the dual innervation corresponds to the dentate line (also called as pectinate line), which is located slightly superior to anal valves or crypts (Fig. 1). Proximal (superior) to the dentate line, the innervation is predominantly splanchnic, derived from S3-S4 spinal segments through the pelvic and prostatic plexus (49). The small segment distal (inferior) to the dentate line that overlies the apex of the prostate is exquisitely sensitive to pain due to the somatic nerve supply through the inferior rectal nerve arising within the pudendal canal. Therefore, the pain in this region is inadequately controlled by the periprostatic nerve block alone and an additional pudendal nerve block or a simpler topical anesthetic application is needed. Location of the dentate line can be confirmed by the rectal sensation test (50) i.e., patient experiences wincing pain when the needle tip is pressed in steps against the anterior rectal wall in a craniocaudal direction. The probe should be placed further above this level so that the biopsy puncture site is above the dentate line.
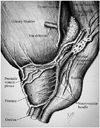
2) Prostate: pain due to prostate biopsy can originate from the capsule or the stroma of the prostate. Most of the pain originates from the capsule of the prostate. Prostatic plexus surrounds the capsule and it has an extensive autonomic innervation (51), rich in nocireceptive splanchnic nerves fibers that originate from sacral 2-5 caudal nerve roots and also from the sympathetic chain via the presacral and hypogastric neural plexuses. The nerve fibers course along the branches of the inferior vesical artery and dorsal vein of the penis to form bilateral neurovascular bundles and are the main sensory supply of the prostate (52, 53). These bundles straddle the posterolateral aspect of the prostate at 5 and 7 o'clock positions, in the angle formed by the seminal vesicle with the base of prostate (54). The branches decussate extensively and ramify between the capsule and the Denonvilliers' fascia (Fig. 2). The apex of the prostate has few peculiar features: i) it is entirely composed of the peripheral zone and its sampling is critical as it is a common conduit for cancer spread. ii) it is the most painful site in the prostate during biopsy since it is located below the dentate line (35). iii) it is the most common site for "missed cancers" on TRUS-guided prostate biopsy as the sampling of this site is often avoided due to the anticipated intense pain (32, 55).
Sites of Injection for the Periprostatic Nerve Block
The sites of injection for the periprostatic nerve block are determined by the location of neurovascular bundles and branches that supply the prostate gland. The completeness of periprostatic nerve block depends on the correct identification of the injection sites. The neurovascular bundles are not directly visualized on ultrasound and their probable location is interpolated from sonographic landmarks. Bilateral symmetrical injections at each site are imperative to achieve complete nerve block due to decussation of fibers in the prostatic plexus. Although some investigators have used a single site of injection, the best results are achieved when these injections are used in combinations. The various sites of injection are as follows:
Bibasal Injections
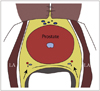
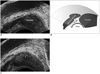
Introduced by Nash et al., this is the most common site of injection (27, 44, 56), when used either alone, or more optimally in combination with other sites of injection. It is a preferred site considering that the major neurovascular bundles of the prostate traverse this site and injection at this site anesthetize a large portion of the prostate gland (Fig. 3). This site is identified by an echogenic triangle of fat at the angle between the seminal vesicle and base of the prostate in the para-sagittal longitudinal scan. The appearance is similar to the snow peak of a mountain and hence it is called "the Mount Everest sign" (Fig. 4).
Apical Injections
Apical injections aim to block the sensitive somatic nerves at the apex of prostate, the most painful site during prostate biopsy (Fig. 5). The site is identified by a smaller echogenic triangle on either side of the apex (57). The drug is deposited between the apex of the prostate gland and the puborectalis muscles (33, 41, 48, 57). The lowest pain score during biopsy is recorded when apical injections are combined with bibasal injections.
Intraprostatic Injections
They cannot be considered as a periprostatic nerve block in a true sense as they do not block the nerve bundles. They are not commonly used although they may anesthetize the anterior prostate which is otherwise suboptimally anesthetized in conventional injection sites. They were used in combination with bibasal injections (59, 60), and their use is now discouraged due to the risk of systemic absorption (2).
Combined Method
The aforementioned injections can be judiciously combined for better results. Typically, the bibasal and biapical injections are combined routinely (44, 56, 61). Occasionally, if an incomplete block is anticipated, as in a bulky prostate, the lateral injections can be given to achieve complete anesthesia (44, 62). Rarely, the intraprostatic instillation can also be added to this combination (60), but this is seldom required and is not recommended.
Technique and Anesthetic Agents Used for the Periprostatic Nerve Block
The technique for the periprostatic nerve block is based on the original method described by Nash et al. (27) in 1996 for basal injections. The procedure can be performed in an out-patient setting, but resuscitation facilities should be available. The same protocol used for pre-procedure preparation of TRUS-guided prostate biopsy is followed. The bladder is emptied before the procedure to prevent urinary retention (33).
The optimal patient position for performing the periprostatic nerve block is the left lateral decubitus position with buttocks at or slightly beyond the side of the table, the left leg in a straight position, and the right leg flexed so as to touch the abdomen. The lithotomy position is an alternative, but it is associated with more discomfort (63).
The ultrasound transducer with the attached biopsy-guide is covered with a disposable sheath that is filled with copious coupling gel. Topical anesthetic gel is applied rectally to facilitate introduction of the probe and to anesthetize the local area. The probe is maneuvered inside the rectum and a preliminary sonographic survey of the prostate is performed. Diagnostic images are recorded at this stage. For bibasal injections, the target is to deliver the drug around the neurovascular bundles coursing on each side of the prostate at the junction with seminal vesicles (Fig. 6). The probe is positioned in the sagittal plane and moved laterally to focus on the triangular echogenic fat ("Mount Everest sign") between the prostate and seminal vesicles (64). With a "side-firing" probe, the optimal position is easily achieved by aligning the probe in the longitudinal plane and rotating it in the parasagittal plane. The "end-firing" probe needs to be swiveled superiorly or inferiorly with its fulcrum against the anus, and therefore, it is essential to position the patient at the edge of the table. The advantage of the "end-firing" probe is that the needle path is along the axis of the probe and the needle pierces the rectum perpendicularly.
Approximately 2.5 mL of lidocaine HCl is deposited at each site under ultrasound guidance using a 23-gauge and 15-cm long needle attached to the calibrated syringe. The appearance of hypoechoic wheal at the needle tip indicates optimal deposition. The wheal gradually disperses along the Denonvillier's fascia (33). Usually, for combined bi-basal and bi-apical injections, a total of 10 mL of the local anesthetic agent provides optimal analgesia although it can be increased to 15 mL (65). For apical or lateral injections, the probe is moved inferiorly or laterally and the local anesthetic is injected in a similar fashion. The whole procedure takes no longer than 5-10 minutes. Biopsy is commenced after a waiting time of 5-10 minutes to allow the local anesthetic to achieve its full effect.
It is important to exclude intravascular position of the needle tip by intermittent aspirations and to complete the drug deposition in several small pushes. The patient's vital signs are closely monitored and the procedure is stopped immediately if early signs of systemic toxicity are noted. All patients are kept under observation for at least two hours and are advised to void before discharge to detect urine retention.
Because of its long-term experience and good safety profile, lidocaine HCl (1-2%) is the most commonly used local anesthetic agent for the periprostatic nerve block. It has a quick onset with an intermediate duration of action. Bupivacaine HCl (0.25%) can be used as an alternative or in combination with lidocaine HCl (66). It has a slower onset but a longer duration of action that provides better post-biopsy analgesia (66, 67).
Efficacy of the Periprostatic Nerve Block
The periprostatic nerve block is the most effective technique to provide local anesthesia for prostate biopsy (3, 51, 62, 68, 69, 70). Although early studies of the periprostatic nerve block for six-core prostate biopsy were equivocal, a large corpus of evidence from randomized controlled studies (27, 33, 48, 56, 61, 71) and meta-analyses (42, 72) proves that the periprostatic nerve block provides substantial pain relief during prostate biopsy. The patients who undergo repeat prostate biopsy usually report significant pain reduction when the periprostatic nerve block is performed (44). Patients are more comfortable and cooperative during the procedure when the periprostatic nerve block is performed and are more agreeable to undergo repeat biopsy. It also reduces the analgesic requirement during the immediate post-procedure period (33).
Combined Perianal-Intrarectal Lidocaine Gel Application and Periprostatic Nerve Block
When used alone, the topical perianal-intrarectal anesthetic gel application is of doubtful efficacy in providing pain relief during prostatic biopsy (26, 42, 73). Nevertheless, it does provide additional pain relief if it is used in combination with the periprostatic nerve block (51, 56) since it reduces the pain caused by probe insertion and needle punctures, which is not controlled by the periprostatic nerve block alone (53). The pain control achieved after the use of this combination is significantly superior to that after any of these techniques used alone, and there is no increase in the overall complication rate (43). Therefore, topical perianal-intrarectal gel application of 10 mL of 2% lidocaine HCl (or equivalent) can be combined with the periprostatic nerve block to achieve better pain control (18, 40, 51).
Complications of the Periprostatic Nerve Block
The periprostatic nerve block is a safe technique that is not accompanied by any major complications (42), if the procedure is carried out after taking proper precautions. The complications associated with the periprostatic nerve block are insignificant (56), and the periprostatic nerve block does not increase the complication rates of a prostate biopsy (33, 41, 43, 56, 74, 75). The low complication rates are attributed to the use of a thin injection needle for anesthetic deposition and to the excellent safety profile of lidocaine.
The periprostatic nerve block requires a few extra needle punctures. The pain due to these punctures is the most common side effect, because the actual biopsy becomes painless due to the block (76).
Vasovagal syncope is rarely reported after the periprostatic nerve block (74, 76). Another concern is the increase in the risk of infection (and septicemia) due to extra punctures in the rectum, but this has not been substantiated by the studies (33, 65, 77) and the risk of infection is negligible as the periprostatic nerve block is performed under antibiotic prophylaxis.
Bleeding complications such as hematuria, hematospermia, and rectal bleeding are not increased after the periprostatic nerve block and are independent of the number of periprostatic nerve block injections (65). Counterintuitively, the incidence of rectal bleeding is reported to be reduced after the periprostatic nerve block, probably due to less discomfort (56).
Potential short-term side-effects due to the blockage of autonomic nerves in the prostatic plexus may include risk of temporary urine retention, urinary incontinence, and transient erectile dysfunction, although they were not found to be significant in the published studies (71).
Degradation of image quality and difficulty in interpretation of diagnostic tests due to artifacts caused by the infiltrated drug and occasional air foci can be avoided by completing the imaging studies prior to periprostatic nerve block injections (61, 76). The radical prostatectomy surgery is unaffected by the periprostatic nerve block (62, 78), and the effect on the subsequent nerve-sparing prostate surgery due to increased vascularization and loss of planes as a result of fibrosis has not been proved during surgery (41, 44, 71).
The most serious complication of the periprostatic nerve block is systemic lidocaine toxicity due to inadvertent intravascular injection into the prostatic venous plexus. Mild lidocaine toxicity causes dizziness, visual disturbance, tinnitus, metallic taste, and diaphoresis. Serious complications such as neurotoxicity and respiratory depression may occur. Lidocaine toxicity can be prevented by performing injections in small steps with repeated aspirations and careful monitoring of the patient during the procedure to detect early signs of systemic toxicity (56). The procedure should be performed in a set up where a resuscitation team and emergency support is available.
Current Recommendations
Periprostatic nerve block provides effective anesthesia with unequivocal efficacy, and it is recommended as a routine adjunct to mitigate pain in patients undergoing TRUS-guided prostate biopsy (79). If the periprostatic nerve block is to be used selectively, identification of patients with high risk of exaggerated pain associated with prostate biopsy is essential. An optimal periprostatic nerve block is achieved by using a combination of bi-basal and apical injections, and if required, augmenting with lateral injections. The topical perianal-intrarectal anesthetic application combined with the periprostatic nerve block ensures optimal pain control during TRUS-guided prostate biopsy (43, 56).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download