Abstract
Objective
The prior functional MRI studies have demonstrated significantly abnormal activity in the bilateral superior temporal gyrus (STG) of anxiety patients. The purpose of the current investigation was to determine whether the abnormal activity in these regions was related to a loss of functional connectivity between these regions.
Materials and Methods
Ten healthy controls and 10 anxiety patients underwent noninvasive fMRI while actively listening to emotionally neutral words alternated by silence (Task 1) or threat-related words (Task 2). The participants were instructed to silently make a judgment of each word's valence (i.e., unpleasant, pleasant, or neutral). A coherence analysis was applied to the functional MRI data to examine the functional connectivity between the left and the right STG, which was selected as the primary region of interest on the basis of our prior results.
Results
The data demonstrated that the anxiety patients exhibited significantly increased activation in the bilateral STG than the normal controls. The functional connectivity analysis indicated that the patient group showed significantly decreased degree of connectivity between the bilateral STG during processing Task 2 compared to Task 1 (t = 2.588, p = 0.029). In addition, a significantly decreased connectivity was also observed in the patient group compared to the control group during processing Task 2 (t = 2.810, p = 0.012).
Anxiety disorder, which is characterized by emotional symptoms of unprovoked anxiety, is a mental disorder commonly observed in clinical practice. Certain pharmacological and cognitive-behavioral treatments effectively ameliorate anxiety symptoms. However, the neural underlying mechanisms are still not well understood. Abnormal emotional hyper-reactivity and the failure of emotional regulation have become the two leading theoretical models that postulate the mechanisms underlying the mood and anxiety disorders (1, 2, 3). Functional magnetic resonance imaging (fMRI) studies have revealed that a variety of anxiety disorders exhibit hyperactivity in multiple cortical and subcortical brain regions, and these are similar to the reactivity exhibited in posttraumatic stress disorder (PTSD) patients (4). For example, hyperactivity of the ventromedial prefrontal cortex, cingulate cortex, amygdala, and insular cortex are frequently observed in neuroimaging studies of PTSD (5, 6). Our previous work demonstrated a significantly increased activity in the bilateral superior temporal gyrus (STG) (7) and an abnormal deactivation of certain brain regions in the default mode network in anxiety patients (8). These observations support the hypothesis that hyper-reactivity and abnormal emotions are exhibited in these regions of the brain.
The communication of information between interconnected brain regions provides the basis for human cognitive processes, and even the simplest cognitive operations are processed through highly distributed neural circuits (9, 10). Recent fMRI studies suggested that many diseases, such as Alzheimer's disease and schizophrenia, are characterized by dysfunctional connectivity (11, 12). On the basis of these findings, we sought to investigate the contribution of dysfunctional connectivity in the neural basis of anxiety disorder and attempted to provide a novel method to examine the dysfunctional emotional regulation of this disease.
Functional connectivity is defined as the temporal correlation between spatially remote neurophysiological events (13). To determine the functional connectivity, simple correlations can be used, which measures the simultaneous coupling between two time series of fMRI data. This has commonly been used to investigate the interactions between the various brain regions at resting state or during the performance of specific tasks (14, 15). However, simple correlation analyses are sensitive to the shape of the regional hemodynamic response function (HRF). Therefore, variability in an individual's HRF across the brain regions can result in unfaithful measures of correlation (16). In contrast, coherence analysis, which estimates the linear, time-invariant relationships between the time series, is less sensitive to inter-regional differences in HRF and can provide detailed information regarding the temporal dynamics of blood oxygen level-dependent signals (17). The current study adopted the coherence analysis for this advantage.
The functional connectivity has traditionally been studied by using the resting state data, but several studies have performed this based on specific task-related activity (15). Our previous task-related study (7) has revealed the interesting results that anxiety patients show significantly increased activation in the left and the right superior temporal gyri, compared to healthy control, while actively listening to emotionally neutral words or threat-related words alternated by silence. Here, we aimed to explore how the functional connectivity changes between the two key brain regions in anxiety patients. We suggest that examining the connectivity between brain regions during task-completing conditions may help to elucidate the dysfunctional emotional regulation of this disease.
We have previously reported the results from this data on activation (7) and deactivation (8). Therefore, only a brief introduction to the subjects, task, and data acquisition is provided here.
Seven male and three female patients with anxiety disorders (mean age 42 years, range 24-50) and ten controls with matching age, sex, and education level participated in the study. The patients were recruited from the Psychosomatic Medicine Department of Tong Ji Hospital of Tong Ji University and met the criteria of Chinese Classification of Mental Disorders for patients with generalized disorder (GAD) and/or panic disorder. All of the subjects were right-handed and free of any other psychiatric symptoms. The subjects were not impaired in auditory or language functions. They had not taken psychotropic medications during the two weeks immediately prior to the participation in this study. All of the subjects provided written consent prior to the study. The study was performed in compliance with the regulations of the Institutional Review Board of our hospital.
All subjects underwent noninvasive fMRI while actively listening to emotionally neutral words (Task 1) or threat-related words (Task 2) alternated by silence. The stimulus words were heard through the noise-attenuating earphones. All words were presented in a pseudorandom order with sixteen alternating blocks based on the task conditions (neutral words in Task 1 and threat-related words in Task 2) and the control condition (resting state with eyes closed in Task 1 and neutral words in Task 2), lasting for about 256 seconds (128 images). For the baseline, resting state was given for 32 seconds to allow the subjects to become familiar with the scanning environments, and this data was discarded from the data analysis.
The subjects were instructed to listen carefully to each stimulus word and to silently make a judgment of the word's valence (i.e., unpleasant, pleasant, or neutral). To avoid learning effect, all the stimuli words in the experiments were played only once. After fMRI scanning, subjects were asked whether they could hear the stimuli words clearly and how good their attention to the task was. No subjects reported of any difficulties with the audibility of the stimuli, their attention to the task, or their performance.
All of the fMRI data were obtained in Tong Ji Hospital of Tong Ji University on a 1.5-T scanner (EDGE ECLIPSE; Marconi Medical Systems, Cleveland, OH, USA) equipped with a prototype fast gradient system for echo-planar imaging. A T2*-weighted, gradient-recalled echo-echo planar imaging sequence was employed for the functional images (echo time, 40 ms; repetition time, 2500 ms; flip angle, 90°; number of excitation, 1; field of view, 24 cm; resolution, 64 × 64 matrix). A total of 144 images were acquired per subject during each 288-second scan.
Image preprocessing and statistical inferences were done using the SPM2 software package (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). The first 16 images (baseline images) in each session were discarded and only the remaining 128 images were used for further analysis. These functional images were first registered to the first time point for head-motion correction. The images were then normalized into Montreal Neurological Institute standard space. Following spatial smooth was performed using a Gaussian kernel of 6 mm3 full width at half maximum, and General linear modeling was performed to observe the activated brain regions in the first level analysis for each subject. The resulting images were then transformed into a unit-normal distribution with an corrected threshold at p < 0.05. A five-voxel threshold was specified to restrict the minimum cluster size. Finally, group level analysis was conducted to obtain brain activation maps. Four statistical t-maps were obtained, corresponding to the Task 1 and Task 2 across the anxiety group and the control group. The activation map was overlapped on the T1 traverse image.
Based on the group t test results, which we have reported previously (7), we identified the left and right superior temporal gyri as seed regions for functional connectivity analysis, because these two regions were significantly activated more in the patient group than in the healthy control group across the Task 1 and Task 2. The regions of interest were then defined individually. The activated voxels with the largest t-values on individual t-map within these two regions were selected as the centers for subject-specific regions of interest (ROIs). Combined with the center's closest 18 neighborhood voxels, individual ROIs were thus defined in squares which were consisted of 19 voxels in total.
We calculated the degree of connectivity between the left and the right STG by using coherence analysis method, by subtracting the measurements taken during the silence from the measurements taken during the neutral words in both control and patient groups (16). These are abbreviated as CN and PN, where C is controls, P is patients, and N is neutral words condition minus silence condition, and as CT and PT where C is controls, P is patients, and T is threat-related words condition minus emotionally neutral words condition. These represent the degree of connectivity between the left and the right STG for controls and patients groups. Subject-specific averaged time series were extracted by averaging the time series of the ROI (19 voxels).
The coherence between the two averaged signals, from region i and region j, is the normalized cross-covariance function in the spectral domain [i.e., cohij(λ) = |fij(λ)| / fii(λ)fjj(λ)]. Here, fij is the cross-spectral density between i and j, fii and fjj are the autospectral density of i and j respectively, and λ represents the frequency. Only the low frequency components were considered (0-0.15 Hz) (16). The band-averaged coherence cohij is thus obtained by averaging the cohij over λ within this band. Moreover, the degree of connectivity (ηij) between region i and region j identifying the changes in functional connectivity is calculated by ηij = e - ξdij. The ξ measures decrease in the strength of the relationship with the decrease in the distance between the two nodes. It is a real positive constant and was fixed to ξ = 2 in this work (18), and dij is the distance between the two regions which was calculated as dij = (1 - cohij) (1 + cohij) (18).
The estimates of functional connectivity were compared between the patient group and the control group using the independent Student t test and between the Task 1 and Task 2 in each group using the paired t test. The SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was considered at p < 0.05.
The differences in the activation patterns and intensities between the patient and control groups across the two tasks have been reported in our previous work (7). Figure 1 depicts the patterns of brain activation during the two experiments for each of the two groups (p < 0.05, corrected). The bright orange represents brain activation during the processing of emotionally neutral words and the blank represents control when no word was heard. The winter blue indicates brain activation during the processing of threatening words and neutral words was the control. The patient group showed significantly greater brain activation in the STG than the normal controls.
The degree of connectivity between the left and the right STG during the performance of these two tasks was calculated across all subjects (0.5636 for CN, 0.555 for CT, 0.5616 for PN, 0.4926 for PT) (Fig. 2). Alterations in the degree of connectivity between brain regions represent changes in the functional interactions between these regions during different tasks. The patient group exhibited significantly decreased PT when compared to PN (t = 2.588, p = 0.029). A significant decrease in the degree of connectivity was observed in the patient group when compared to the control group, during the alternation of threat-related words with emotionally neutral words (t = 2.810, p = 0.012) (Fig. 2). These results suggest a decrease in the interaction between these two brain regions during the processing of an anxiety-provoking word compared to a neutral word in anxiety patients.
The prevalence of anxiety disorder is growing dramatically, but the neural mechanisms underlying this disease remain unknown. The emotional dysregulation hypothesis (19, 20, 21) suggests that anxiety involves exaggerated emotional experiences, a poor understanding of emotions (e.g., the inability to identify discrete emotions and use them as a source of knowledge), and negative reactivity to one's emotional state (e.g., fear of emotion). This model has been thought to reasonably clarify this neural mechanism, and it generalizes the abnormal emotional hyper-reactivity hypothesis and a failure of emotional regulation hypothesis, both of which may play a crucial role in the underlying neural mechanisms of anxiety disorders (1, 2, 3). These emotional abnormalities can provoke fear and anxiety in patients. Similarly, hyperactivity of the ventromedial prefrontal cortex and cingulate cortex has been observed in the patients with PTSD (5, 6) and other anxiety disorders (4). Our previous work revealed hyperactivity of the bilateral STG in anxiety patients (7). These findings support the notion that an abnormal emotional hyper-reactivity mechanism may exist in anxiety patients.
The transfer of information between interconnected brain regions provide the basis for human cognitive processes, and even the simplest cognitive operations are processed through highly distributed neural circuits (9, 10). Functional connectivity methods, such as the coherence analysis approach used in the current study, can characterize the interactions between the brain regions, thus providing a potential novel approach to investigate the hypothesis that a failure of emotional regulation is characteristic of anxiety patients. Our current results demonstrate that anxiety patients exhibit a significant decrease in the functional connectivity between the left and the right STG, during the processing of anxiety-inducing words relative to neutral words when compared with the processing of neutral words relative to a blank. The anxiety patients also exhibited decreased connectivity between these two regions, compared to normal controls, during the processing of anxiety-provoking words relative to neutral words. These observations are very interesting. A prior study with task-related fMRI data and coherence functional connectivity analysis revealed that the brain areas that showed increased functional connectivity during both tasks were the same as those that showed increased activity (16). This finding suggests that the functional connectivity between the brain regions appears to synchronize with the activity of those brain regions. The current study revealed a significantly increased activity in the right and the left STG but a decreased functional connectivity between these two regions, a result that is in contrast with the findings in normal subjects (16). We speculate that the increased activity of the bilateral STG but the decreased functional connectivity between the right and the left STG suggests an abnormal regulation of coherence between these two regions. This may contribute to the neural basis of anxiety in these patients and provide new insight into this disease. Research on anxiety disease using functional connectivity has been relatively rare (22, 23). Hahn et al. (23) observed hyperactivity of the amygdala during the performance of an emotional task and decreased functional coupling of the left amygdala with the medial orbitofrontal cortex and the posterior cingulate cortex/precuneus. This finding is consistent with our results. Etkin et al. (22) reported that GAD patients exhibited increased connectivity within the frontoparietal executive control network but decreased connectivity with the insula and cingulate-based salience networks. The authors proposed that the increased connectivity within the frontoparietal executive control networks may be involved in a compensatory executive control network, which would be consistent with the cognitive theories of GAD.
The primary objective of the current study was to investigate functional connectivity across brain regions, and this study primarily focused on the STG. Our previous results also demonstrated abnormal hyperactivity in other regions, such as the prefrontal region, in anxiety patients (7); however, these abnormal activations were located unilaterally in the right hemisphere. The STG has traditionally been recognized as the auditory cortex. However, a growing number of data indicates that this region is also closely engaged in the processing of social information, and it participates in high level cognitive functioning in humans and other animals (24, 25). The auditory cortical areas may be particularly involved in the higher cognitive (mnemonic and attentional) processing of fearful experiences in rats (24). In addition, pediatric GAD subjects exhibited significantly larger volumes of white and gray matter in the STG than control subjects (25). These observations strongly support a role for the STG in higher cognitive functioning and its involvement in anxiety disorders.
Several limitations of this study should be considered. For example, the current study included a relatively small sample size, and the correlations between the altered connectivity and the behavioral measures have not been explored. Future studies should be conducted to correlate the functional data with behavioral measures.
In conclusion, the study results suggest that the anxiety patients exhibit increased activity of the STG but decreased functional connectivity between the left and right STG, which may reflect the underlying neural abnormality of anxiety disorder and provide new insights into this disease.
Figures and Tables
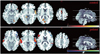 | Fig. 1
Statistical t-maps reveal brain activity during processing of emotionally neutral words relative to no words (hot orange) and during processing of threatening words relative to neutral words (winter blue) in anxiety patients and normal controls. Anxiety patients exhibited significantly greater response in bilateral superior temporal gyrus than normal controls during processing of threatening words relative to neutral words. |
 | Fig. 2
Differences in degree of connectivity, η, is shown between left and right STG, during two tasks in anxiety patients and normal controls. Anxiety patients exhibited significant decrease in degree of connectivity during processing of threatening words relative to neutral words and during processing of emotionally neutral words relative to no words, compared to normal controls. *p < 0.05. STG = superior temporal gyrus |
Acknowledgments
The authors thank American Journal Experts for their helpful comments and extensive language editing.
References
1. Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry. 2002; 51:92–100.
2. Hermann C, Ofer J, Flor H. Covariation bias for ambiguous social stimuli in generalized social phobia. J Abnorm Psychol. 2004; 113:646–653.
3. Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In : Gross JJ, editor. Handbook of Emotion Regulation. New York, NY: Guilford Press;2007. p. 542–559.
4. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007; 164:1476–1488.
5. Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004; 4:275–284.
6. Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res. 2006; 40:709–729.
7. Zhao XH, Wang PJ, Li CB, Wang JH, Yang ZY, Hu ZH, et al. Prefrontal and superior temporal lobe hyperactivity as a biological substrate of generalized anxiety disorders. Zhonghua Yi Xue Za Zhi. 2006; 86:955–960.
8. Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007; 63:373–378.
9. Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003; 19(2 Pt 1):466–470.
10. Sporns O. Networks of the Brain. Cambridge, MA: MIT Press;2010. p. 179–201.
11. Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008; 131(Pt 4):945–961.
12. Delbeuck X, Collette F, Van der Linden M. Is Alzheimer's disease a disconnection syndrome? Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia. 2007; 45:3315–3323.
13. Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993; 13:5–14.
14. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995; 34:537–541.
15. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002; 15:247–262.
16. Sun FT, Miller LM, D'Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004; 21:647–658.
17. Müller K, Lohmann G, Bosch V, von Cramon DY. On multivariate spectral analysis of fMRI time series. Neuroimage. 2001; 14:347–356.
18. Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004; 22:63–71.
19. Turk CL, Heimberg RG, Luterek JA, Mennin DS, Fresco DM. Emotion dysregulation in generalized anxiety disorder: a comparison with social anxiety disorder. Cognit Ther Res. 2005; 29:89–106.
20. Mennin DS, Turk CL, Heimberg RG, Carmin CN. Regulation of emotion in generalized anxiety disorder. In : Reinecke MA, Clark DA, Beck A, editors. Cognitive Therapy over the Lifespan: Theory, Research, and Practice. New York: Cambridge University Press;2003. p. 60–89.
21. Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clin Psychol Sci Pract. 2002; 9:85–90.
22. Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009; 66:1361–1372.
23. Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011; 56:881–889.
24. Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997; 19:613–624.
25. De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002; 51:553–562.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download