Abstract
Objective
The purpose of this study was to determine the incidence and risk factors of infections associated with implantable venous access ports (IVAPs).
Materials and Methods
From August 2003 through November 2011, 1747 IVAPs were placed in our interventional radiology suite. One hundred forty four IVAPs were inserted in patients with hematologic malignancy and 1603 IVAPs in patients with solid tumors. Among them, 40 ports (23 women and 17 men; mean age, 57.1 years; range, 13-83) were removed to treat port-related infections. We evaluated the incidence of port-related infection, patient characteristics, bacteriologic data, and patient progress. Univariable analyses (t test, chi-square test, and Fisher's exact test) and multiple logistic regression analyses were used to determine the risk factors for IVAP related infection.
Results
Overall, 40 (2.3%) of 1747 ports were removed for symptoms of infection with an incidence rate of 0.067 events/1000 catheter-days. According to the univariable study, the incidences of infection were seemingly higher in the patients who received the procedure during inpatient treatment (p = 0.016), the patients with hematologic malignancy (p = 0.041), and the patients receiving palliative chemotherapy (p = 0.022). From the multiple binary logistic regression, the adjusted odds ratios of infection in patients with hematologic malignancies and those receiving palliative chemotherapy were 7.769 (p = 0.001) and 4.863 (p = 0.003), respectively. Microorganisms were isolated from 26 (65%) blood samples, and two of the most causative organisms were found to be Staphylococcus (n = 10) and Candida species (n = 7).
The major complications of implantable venous access ports (IVAPs) placement include infection, thrombosis, catheter obstruction, extravasation, and catheter migration (1). Among these, IVAP-related infection is the most common complication that results in device removal (1, 2). Fischer et al. (2) reported that 46.2% of IVAP removal was for managing infectious complication, which was much higher than the rates for thrombosis or dysfunction. Biffi et al. (3) analyzed the costs of IVAP-related complications and found that the treatment of IVAP-related bacteremia had the highest cost.
Several authors studied the factors that increase the infectious port complications, and one of the most significant factors was the hematologic malignancy. Samaras et al. (4) reported that the port-associated infections are mostly observed in younger patients with hematologic malignancy, and assumed that intensive chemotherapy and prolonged neutropenia might be responsible for the results. Another recent study showed that outpatient placement of the IVAPs reduced the infection rate (5). To the best of our knowledge, there are only a few studies systematically analyzing the risk factors of IVAP related infection, with a large number of patients. The purposes of this study were to determine the incidence of the infectious complications in IVAPs placed under sonographic and fluoroscopic guidance, and to investigate the statistically significant risk factors of infectious complications. We also determined the microorganisms that commonly cause IVAP-related infections.
This retrospective study was approved by the Institutional Review Board of our hospital, and the requirement for patient informed consent was waived. From August 2003 through November 2011, we placed 1747 ports under sonographic and fluoroscopic guidance in our interventional radiology suites. Among 1747 patients of 1042 women and 705 men (mean age ± 1 standard deviation, 57.2 ± 13 years; range, 13-90 years), 1603 had solid organ 144 had hematologic malignancies, and all of the patients required IVAP placement for long-term administration of chemotherapy. Of those, 1203 patients received the procedure while undergoing inpatient management, whereas 544 received the procedure as an outpatient procedure.
All IVAPs were placed by one of the two interventional radiologists over the study period. The procedures were carried out under aseptic conditions and the prophylactic antibiotics were not used. The following equipment were used: Healthport (8-Fr, Baxter Healthcare SA, Zurich, Switzerland), Celsite (6.5-Fr or 8.5-Fr, B. Braun Medical, Boulogne Cedex, France), Vaxel port (8-Fr, Navylist Medical Inc., Marlborough, MA, USA), X-port (8-Fr, Bard Access Systems Inc., Salt Lake City, UT, USA), and Vital port (6.5-Fr, Cook Inc., Bloomington, IN, USA).
Before skin disinfection and sterile draping, we performed neck ultrasound to select the venous access site and to confirm the patency and size of the targeted vein. In 1441 cases, the right internal jugular vein (RIJV) was punctured under sonographic guidance, after subcutaneous injection of local anesthesia. The left internal jugular vein was punctured in 304 patients, who had any of the following conditions: right breast cancer, sonographically proven RIJV thrombosis, or any skin lesion around the procedure site interfering with puncture and port placement. In the remaining two patients, the left and the right axillary veins were selected. After making a 0.5-cm stab incision at the venipuncture site, a 0.018-inch microwire was fluoroscopically advanced to the cavoatrial junction using a micropuncture set (Micronitinol rapid access kit, Access Point Technologies, Rogers, MN, USA).
Under local anesthesia, horizontal incision of 2-3 cm was made below the clavicle, following the direction of skin fold, and a blunt dissection of subcutaneous layer was performed to create a pocket. After controlling the bleeding, the port catheter was tunneled from the pocket to the puncture site using a tunneling device. We measured the length of a 0.018-inch microwire under fluoroscopy to ensure the accurate placement of the catheter tip. The microwire was exchanged to a 0.035-inch guidewire, over which, the serial dilation of the venipuncture tract was performed which was followed by the placement of a peel-away sheath. Through the sheath, the catheter was introduced into the accessed vein and the catheter tip was placed just distal to the cavoatrial junction under fluoroscopic guidance. The port chamber was fixed to the pectoral fascia with absorbable suture (3-0, Surgifit, Ailee Co., Ltd., Busan, Korea). The port patency was ascertained by aspirating a small amount of blood. Then, 300 units of heparin-saline solution was instilled into the port chamber and the catheter lumen. After flushing the port pocket with a Cefamezine (cefazolin sodium, Dong-A Pharmaceutical Co., Seoul, Korea) 1 g mixture, subcutaneous layer was sutured with absorbable suture material and skin layers of pocket and venous access site were sutured with non-absorbable material (4-0, Blue nylon, Ailee Co., Ltd., Busan, Korea) materials. A final fluoroscopic image documented the correct positioning of the catheter tip and the satisfactory catheter course without acute angulation. The needle access of IVAPs was permitted from the day of the port placement, according to the patient's chemotherapy schedule. The wound dressing was done on the third or fourth day, and suture removal was done on the seventh or eighth day after the procedure. None of the patients received systemic antibiotics after the procedure.
We retrospectively evaluated the incidence of port-related infections, patient demographic factors, bacteriologic data, and patient progress by reviewing the medical records. The local infections include phlebitis, tunnel infection, and exit site infection. The patients with erythema, warmth, induration, and pain along the catheterized vein or around the catheter exit site were enrolled in the local infection group. The wound dehiscence, pus discharge, skin discoloration with fistula formation, and skin ulceration were also assumed as the clinical signs of IVAP-related local infection. The systemic infection, which is a synonym for bloodstream infection, was defined by the following conditions: bacteremia or fungemia in a patient with intravascular device, where more than one positive blood culture results were obtained from the peripheral vein; and clinical manifestations of infection (e.g., fever, chills) with no apparent source for bloodstream infection (6). The patients with suspected catheter-related infection were also included in systemic infection group; these patients had microbiological results that are insufficient to diagnose catheter-related bloodstream infection, but the demonstrated apparent symptoms of infections. The immediate infections were defined as the infections occurring within 30 days of IVAP placement (4, 7). Other infections were classified as the delayed infections.
For all patients with signs and symptoms of catheter-related infections, we performed aerobic and anaerobic microorganism culture with whole blood samples before explantating the port device. The catheter tips of the removed IVAPs were placed on agar plates and delivered to the laboratory for microbiology study. When local infection was suspected, we obtained a wound swab culture.
For calculating the incidence rate (events per 1000 catheter days), the duration of IVAP catheter use was incorporated (Table 1). A total of 345 patients had their IVAPs removed during the study period. The reasons for IVAP removal were chemotherapy termination in 270 patients and development of complications in 75 patients; the complications included thrombosis, skin necrosis due to catheter leakage, migration, occlusion, and intractable pain. In 566 patients who still had devices in place, we calculated the duration of IVAP use from the cutoff day of February 11, 2012. For the patients who died or those who were lost to follow-up before the cutoff day (n = 836), we used the last day of their medical records instead of the day of IVAP removal.
Statistical analysis was performed with IBM SPSS Statistics 20.0 (SPSS IBM, New York, NY, USA). To determine risk factors for infectious complications, univariable analyses (using the t test, chi-square test, and Fisher's exact test) and multiple logistic regression analyses were used. The age, gender, patient location (i.e., inpatient vs. outpatient), puncture site, performance status, types of malignancy, and the nature of chemotherapy (i.e., palliative vs. curative-intent) were analyzed. Variables were included in the multivariable analysis if the p value at the univariable analysis was less than 0.1, with the exception of the patients' location. The patients' location was considered only for the univariable analysis, because it is likely to represent multiple individual factors collectively (Table 2) and would hinder the analysis of individual factors due to multicollinearity. We used backward method of binary logistic regression analysis. P values < 0.05 were considered statistically significant.
In 345 cases of IVAP removal, the most common indication for removal was termination of chemotherapy and the second most common was to treat suspected infection. Overall, 45 (2.58%) of 1747 IVAPs were explanted to treat suspected infection. Five patients were proven to have incidental infections unrelated to IVAP (i.e., pneumonia or urinary tract infection). The calculated incidence rate of IVAP-related infection was 0.067 events/1000 catheter-days. For the infection group (n = 40; 23 women and 17 men; mean age, 57.1 years; range, 13-83), the median patency of inserted IVAPs was 143 days (range 18-827 days). Of 40 patients who had symptoms and signs of infection, 31 had systemic illness and 8 had local infection. A patient with breast cancer had signs of both systemic and local infection. Six patients had immediate infections (median patency, 24; range, 18-25 days), and 34 had delayed infection (median patency, 158; range, 31-827 days) (Table 3).
We removed 7 devices from patients with hematologic malignancy and 33 from patients with solid organ malignancy. The infection incidence rate per 1000 catheter days was 0.116 for hematologic malignancy and 0.061 for solid organ malignancy. The proportion of port-related infection was higher in the patients with hematologic disease than in the patients with solid organ tumors (4.9% and 2.1%, respectively, p = 0.041) (Table 4). However, the incidence did not correlate with white blood cell counts at the time of IVAP placement.
We performed the univariable comparison between the infection group and the control group, and the results are shown in Table 5. Multivariable binary logistic regression analysis revealed that hematologic malignancy and palliative chemotherapy were independent risk factors of IVAP-related infection (Table 6). The adjusted odds ratio (OR) of infectious complication for hematologic malignancy versus solid organ malignancy was 7.769 (95% confidence interval, 2.356 to 25.615). The adjusted OR for palliative chemotherapy versus adjuvant, neoadjuvant, and curative chemotherapy was 4.863 (95% confidence interval, 1.726 to 13.700).
We also compared the systemic infection and local infection groups, and there were no statistically significant differences in all variables (p values = 0.102 to 0.703).
The microorganisms were isolated from 26 (65%) blood samples, and the common causative microorganisms were found to be Staphylococcus species (n = 10), Candida species (n = 7), and non-tuberculosis Mycobacterium (n = 2). Other microorganisms such as Escherichia coli, Acinetobacter baumannii, Klebsiella pneumonia, etc. were isolated from the rest of the 6 blood samples (Table 7). In addition, a microbiological study of catheter tip was shown to be positive in 9 cases, with microorganisms that were the same as the blood culture studies. The wound culture was performed in the patients with clinically suspected localized infection without any detection of microorganisms (Table 7). After IVAP removal, antibiotics were administered according to the results of microbiology and antibiotic sensitivity tests.
The total incidence of IVAP-related infection was 0.067 events/1000 catheter days. The previously reported IVAP-related infection rates were considerably higher (0.16 to 0.35 events/1000 port days) (4, 8, 9, 10, 11, 12, 13). However, the lower incidence has been reported on recent studies, indicating that a large number of cases, well-experienced procedures, and management had lowered the infection. Ahn et al. (14) reported a lower infectious complication rates (0.64%, 0.018 per 1000 catheter days) and they emphasized the clinical importance of the infectious complication as a major cause of prolongation of hospitalization. They strictly defined the catheter-related bloodstream infection as when a blood culture is positive without other identifiable sources of infection, and if the clinical signs resolve within 48 hours after port explantation.
Demographic factors, such as the relatively small number of hematologic malignancy patients, might contribute to lower infection rates. The proportion of hematologic malignancy patients was relatively small in our study (8.2%, 144/1747) compared to other studies (27-36%) (4, 8, 9, 10, 11, 12, 13). Since hematologic malignancy is highly associated with catheter-related bloodstream infection, this difference could have led to selection bias in our study (4). However, the incidence rate of infection in hematologic malignancy patients (0.116 events/1000 catheter-days) was not higher than other studies. A larger proportion of outpatients might be related to the low infection rate.
The incidence of infection was significantly higher in hematologic malignancy patients. Hematologic malignancy was more strongly related to the delayed bloodstream infections rather than immediate local infections. This observation was similar to the previous studies (2, 4,13). Impaired immunity caused by both the disease itself and the use of immunosuppressants after bone marrow transplantation would result in the difference of outbreak of infection. More intensive chemotherapy schedule for hematologic malignancies compared to that of solid tumors can contribute to increased infection (15).
The IVAP-related infection rate was significantly higher when ports were placed in the inpatients (p = 0.016), although it may not be a true risk factor for infection. Pandey et al. (5) argued that the outpatient port placement is associated with a decreased risk of infection, and it is possibly owing to the frequent needle access and exposure to nosocomial infection of the inpatients. Further studies with strictly controlling intervariable collinearity and confounding factors should be followed to clarify the cause-and-effect relationship of the outpatient port placement and the infection.
Staphylococcus and Candida species were the two most commonly isolated organisms from the patients with IVAP-related infections. For these infections, removal is always necessary, while the system can be successfully maintained for infections with coagulase-negative Staphylococcus, Corynebacterium jeikeium, or Pseudomonas aeruginosa (16). If a patient presents with fever and a negative culture study, the decision for removing the port device is difficult. However, in our study, 14 patients had fever with negative results of microbiologic studies, and they experienced symptom relief after IVAP removal and antibiotic administration.
Some authors previously analyzed the risk factors affecting catheter-related infection and suggested some strategies for reducing the infection rate. The young age and hematologic malignancy are known to be highly associated with catheter-related bloodstream infections (4,17,18). However, patient age was not associated with infection in our study. The catheter-related thrombosis was also known to increase the risk of systemic catheter-associated infections, although no thrombotic complications occurred in our infection group (19, 20). Fischer et al. (2) reported that the patients with ongoing chemotherapy and those with recurrent IVAP placement experienced infections far more frequently than the others. They also reported that the breast cancer patients are less likely to experience catheter-related infection than the patients with other malignancies. Lebeaux et al. (21) reported patients' overall conditions (comorbidities and performance status) and elevated C-reactive protein levels were associated with unfavorable clinical outcomes after an IVAP-related infection. In our study, the performance status did not significantly correlate with the incidence of IVAP-related infection. But, we found that the patients receiving palliative chemotherapy were more susceptible to infection than others, probably related to the prolonged use of IVAP due to frequent chemotherapy schedules.
The use of antibacterial-impregnated catheters was limited to catheter-related bloodstream infections, but further studies may be needed to clarify the cost effectiveness of those devices. The periprocedural antibiotic prophylaxis is controversial; some randomized prospective studies from surgical teams suggested that IVAPs may be implanted without any antibiotic prophylaxis when following strict methods of pre- and postoperative care, especially in patients with solid organ malignancy (22,23). The education and training programs for the patients and healthcare providers involved in the insertion and maintenance of catheters will be helpful for reducing the infection rate (24).
The limitations of our study were as follows. First, it was a retrospective, single-centered study, and the retrospective collection of periprocedural clinical data was frequently impossible due to lack of documents. Second, a considerable number of follow-up loss of patients was not excluded from our study, which possibly could have resulted in over- or undercalculation of incidence. Third, the incidence of infection could have been underestimated, if the clinically undetected catheter-related bloodstream infection, the rapidly progressing catheter-related fatal bloodstream infection, or the medically treated infections were not notified to the intervention radiologists. Fourth, some of the infections that we have reported may have resulted from other sites of unrecognized infections. Fifth, further retrospective analyses of periprocedural laboratory data were impossible due to the heterogeneity of the time intervals between the procedures and the laboratory examinations; the data including the periprocedural data after chemotherapy were not obtained, which may considerably have affected the results.
In conclusion, the underlying hematologic malignancy and the state of receiving palliative chemotherapy were the independent risk factors of IVAP-related infection.
Figures and Tables
Table 5
Univariable Comparison between Infection Group and Control Group
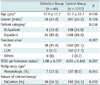
Note.-All values in parentheses are in percentile. ECOG performance status is written in average ± standard deviation. Statistical tests were performed with *t test, †Chi-square test, and ‡Fisher's exact test. ECOG = Eastern cooperative oncology group, LIJV = left internal jugular vein, RIJV = right internal jugular vein
References
1. Narducci F, Jean-Laurent M, Boulanger L, El Bédoui S, Mallet Y, Houpeau JL, et al. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol. 2011; 37:913–918.
2. Fischer L, Knebel P, Schröder S, Bruckner T, Diener MK, Hennes R, et al. Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol. 2008; 15:1124–1129.
3. Biffi R, de Braud F, Orsi F, Pozzi S, Mauri S, Goldhirsch A, et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol. 1998; 9:767–773.
4. Samaras P, Dold S, Braun J, Kestenholz P, Breitenstein S, Imhof A, et al. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008; 74:237–244.
5. Pandey N, Chittams JL, Trerotola SO. Outpatient placement of subcutaneous venous access ports reduces the rate of infection and dehiscence compared with inpatient placement. J Vasc Interv Radiol. 2013; 24:849–854.
6. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 49:1–45.
7. Gebauer B, El-Sheik M, Vogt M, Wagner HJ. Combined ultrasound and fluoroscopy guided port catheter implantation--high success and low complication rate. Eur J Radiol. 2009; 69:517–522.
8. Biffi R, Corrado F, de Braud F, de Lucia F, Scarpa D, Testori A, et al. Long-term, totally implantable central venous access ports connected to a Groshong catheter for chemotherapy of solid tumours: experience from 178 cases using a single type of device. Eur J Cancer. 1997; 33:1190–1194.
9. Brown DF, Muirhead MJ, Travis PM, Vire SR, Weller J, Hauer-Jensen M. Mode of chemotherapy does not affect complications with an implantable venous access device. Cancer. 1997; 80:966–972.
10. Kock HJ, Pietsch M, Krause U, Wilke H, Eigler FW. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg. 1998; 22:12–16.
11. Lyon RD, Griggs KA, Johnson AM, Olsen JR. Long-term follow-up of upper extremity implanted venous access devices in oncology patients. J Vasc Interv Radiol. 1999; 10:463–471.
12. Wolosker N, Yazbek G, Nishinari K, Malavolta LC, Munia MA, Langer M, et al. Totally implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J. 2004; 122:147–151.
13. Caers J, Fontaine C, Vinh-Hung V, De Mey J, Ponnet G, Oost C, et al. Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer. 2005; 13:325–331.
14. Ahn SJ, Kim HC, Chung JW, An SB, Yin YH, Jae HJ, et al. Ultrasound and fluoroscopy-guided placement of central venous ports via internal jugular vein: retrospective analysis of 1254 port implantations at a single center. Korean J Radiol. 2012; 13:314–323.
15. Gapany C, Tercier S, Diezi M, Clement C, Lemay K, Joseph JM. Frequent accesses to totally implanted vascular ports in pediatric oncology patients are associated with higher infection rates. J Vasc Access. 2011; 12:207–210.
16. Fätkenheuer G, Buchheidt D, Cornely OA, Fuhr HG, Karthaus M, Kisro J, et al. Central venous catheter (CVC)-related infections in neutropenic patients--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol. 2003; 82:Suppl 2. S149–S157.
17. Lebeaux D, Zarrouk V, Leflon-Guibout V, Lefort A, Fantin B. [Totally implanted access port-related infections: features and management]. Rev Med Interne. 2010; 31:819–827.
18. Teichgräber UK, Kausche S, Nagel SN. Evaluation of radiologically implanted central venous port systems explanted due to complications. J Vasc Access. 2011; 12:306–312.
19. Aitken DR, Minton JP. The "pinch-off sign": a warning of impending problems with permanent subclavian catheters. Am J Surg. 1984; 148:633–636.
20. Hinke DH, Zandt-Stastny DA, Goodman LR, Quebbeman EJ, Krzywda EA, Andris DA. Pinch-off syndrome: a complication of implantable subclavian venous access devices. Radiology. 1990; 177:353–356.
21. Lebeaux D, Larroque B, Gellen-Dautremer J, Leflon-Guibout V, Dreyer C, Bialek S, et al. Clinical outcome after a totally implantable venous access port-related infection in cancer patients: a prospective study and review of the literature. Medicine (Baltimore). 2012; 91:309–318.
22. Di Carlo I, Toro A, Pulvirenti E, Palermo F, Scibilia G, Cordio S. Could antibiotic prophylaxis be not necessary to implant totally implantable venous access devices? Randomized prospective study. Surg Oncol. 2011; 20:20–25.
23. Karanlik H, Kurul S, Saip P, Unal ES, Sen F, Disci R, et al. The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg. 2011; 202:10–15.
24. Gastmeier P, Geffers C. Prevention of catheter-related bloodstream infections: analysis of studies published between 2002 and 2005. J Hosp Infect. 2006; 64:326–335.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download