Abstract
Objective
The purpose of our study was to assess the feasibility of performing percutaneous radiologic gastrostomy (PRG) in patients who had undergone partial gastrectomy and to evaluate factors associated with technical success.
Materials and Methods
Nineteen patients after partial gastrectomy, who were referred for PRG between April 2006 and April 2012, were retrospectively analyzed. The remnant stomach was punctured using a 21-gauge Chiba-needle. A single anchor was used for the gastropexy and a 12-Fr or 14-Fr gastrostomy tube was inserted. Data were collected regarding the technical success, procedure time, and presence of any complications. Univariable analyses were performed to determine the factors related to the technical success.
Results
Percutaneous radiologic gastrostomy was technically successful in 10 patients (53%), while a failed attempt and failure without an attempt were observed in 5 (26%) and 4 (21%) patients, respectively. Percutaneous radiologic jejunostomy was successfully performed in 9 patients who experienced technical failure. In the 10 successful PRG cases, the mean procedure time was 6.35 minutes. Major complications occurred in 2 patients, tube passage through the liver and pneumoperitonum in one and severe hemorrhage in the other. The technical success rate was higher in patients with Billroth I gastrectomy (100%, 6/6) than in patients with Billroth II gastrectomy (31%, 4/13) (p = 0.011).
Percutaneous gastrostomy is widely recognized as a safe procedure for providing access for enteral nutrition in patients with unsafe or impossible condition for oral intake, and it has a low risk of complications (1, 2, 3, 4, 5, 6, 7, 8). Percutaneous gastrostomy procedures have been reported to be used in patients who have undergone partial gastrectomy (9, 10, 11, 12, 13, 14). The main concern regarding the percutaneous gastrostomy in patients after partial gastrectomy is the high subcostal position of the remnant stomach that precludes the performance of fluoroscopically or endoscopically guided procedures (9, 13).
Percutaneous radiologic gastrostomy (PRG) is commonly indicated for patients with obstructing pharyngeal or esophageal carcinoma, with contraindications for percutaneous endoscopic gastrostomy (5, 15). This procedure offers the benefit of avoiding the drawbacks and costs of endoscopy and surgery (16). Furthermore, the PRG technique generally has the advantages of requiring only a short amount of time as well as a high technical success rate. To date, there have been case reports only regarding the percutaneous gastrostomy performed under fluoroscopic guidance in patients with a history of partial gastrectomy (9, 12). Therefore, the purpose of our study was to determine the feasibility of performing PRG in patients who had undergone partial gastrectomy and to evaluate the factors associated with technical success.
Informed consent for PRG was obtained from each patient. This retrospective study was approved by our Institutional Review Board.
A consecutive series of 19 patients who were referred for PRG during a period of six years, between April 2006 and April 2012, were included as the sample for this study. Sixteen patients were male with age range of 57 to 87 years (median, 72 years). The indications for PRG included dysphagia, or the inability to swallow, due to organic brain damage (n = 5), advanced esophageal cancer (n = 4), chronic obstructive pulmonary disease (n = 3), head and neck cancer (n = 3), Parkinson's disease (n = 2), and generalized poor medical condition (n = 2).
All patients had undergone partial gastrectomy using either a gastroduodenostomy (Billroth I procedure) (n = 6) or a gastrojejunostomy (Billroth II procedure) (n = 13) for gastric cancer (n = 16) and benign diseases such as gastric perforation (n = 2) and gastric ulcer (n = 1). The interval between the surgery and the attempt to perform PRG was from 3 to 625 months (median, 118 months).
A nasogastric tube was used to inflate the remnant stomach with approximately 200-300 mL of air. The anteroposterior and lateral radiographs were obtained in order to confirm that the remnant stomach lay in contact with the anterior abdominal wall with no interposing bowel and that the transverse colon had been inferiorly deflected.
A detailed description of the PRG technique has been provided in a previous study (9). Under fluoroscopic guidance, an appropriate puncture site was selected overlying the lower body of the remnant stomach. A 21-gauge Chiba needle (Cook, Bloomington, IN, USA) was then advanced into the insufflated remnant stomach toward the fundus. After confirming the needle position by contrast (Omnipaque 300, GE Healthcare, Cork, Ireland) injection, a 0.018-inch guide wire (Cook, Bloomington, IN, USA) was passed into the stomach. The Chiba-needle was then exchanged for a 6-Fr Neff catheter (Cook, Bloomington, IN, USA). A Cope suture anchor (Cook, Bloomington, IN, USA) was then deployed into the stomach lumen through the Neff catheter using a 0.035-inch super-stiff guide wire (Cook, Bloomington, IN, USA) for gastropexy. Once the stomach was anchored to the abdominal wall, the puncture site was serially dilated over the 0.035-inch super-stiff guide wire, in order to allow placement of a 12-Fr or 14-Fr diameter locking loop catheter (Cook, Bloomington, IN, USA) (Fig. 1).
All patients underwent a fluoroscopic examination by administering contrast (Omnipaque 300, GE Healthcare) via the gastrostomy tube on the first day and on the first week following its placement, in order to confirm that the gastrostomy tube was correctly placed. The feeding tube was started once the initial follow-up contrast study demonstrated the absence of leakage and other complications related to the tube placement. The external portion of the gastropexy suture was cut at the time of the one-week follow-up.
The data regarding the following variables were collected: technical result of the procedure, causes of technical failures, the procedure time, and any complications occurring within 30 days. The patients were divided into three groups according to the technical result of the procedure, for example, technical success, failed attempt, and failure without attempt. The failed attempt and failure without attempt corresponded to the technical failure.
The technical success was defined as a correct positioning of the feeding tube in the remnant stomach, which is fluoroscopically confirmed using contrast medium and documented at the end of the procedure. A failed attempt was defined as unsuccessful positioning of the feeding tube due to a failure of puncturing the remnant stomach. A failure without attempt was defined as an aborted procedure without the attempt for puncturing, because no optimal puncture site was found under fluoroscopy.
The procedure time was defined as the time length between lidocaine skin infiltration and confirmation of the feeding catheter placement within the stomach by contrast administration. Major complications were defined as the conditions that are life-threatening, causing gastrostomy malfunction, or requiring additional interventional procedures. Minor complications were defined as the conditions requiring only minimal medical management or local wound care (1).
Univariable analyses were performed using the Student t test and the Fisher's exact test for continuous data and categorical data, respectively. The following data were evaluated: age, gender, types of gastric surgery, indications for PRG, duration between the surgery and PRG, complications, and technical success/failure. A two-sided p value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 19; Armonk, NY, USA).
The technical success of PRG, using the modified Chiba-needle technique and single gastropexy under fluoroscopic guidance, was achieved in 10 (53%) out of 19 patients. In the successful cases, tenting of the remnant stomach wall was clearly seen during the advancement of the Chiba needle. The mean procedure time was 6.35 minutes in the 10 patients with technical success. In 9 patients with technical failure, there was a failed attempt and failure without attempt in 5 (26%) and 4 (21%) patients, respectively.
The puncturing of the remnant stomach was attempted in 79% (15/19) of all referred patients, and the technical success rate in those attempted cases was 67% (10/15). In the failed attempt group (n = 5), the causes were positioning of the remnant stomach under the lower left rib cage (n = 4) or presence of bowel anterior to the remnant stomach (n = 1). In the failure without attempt group (n = 4), bowel was seen anterior to the remnant stomach on the anteroposterior and lateral radiographs obtained immediately before the procedure in all patients. PRJ was successfully performed in the 9 patients who experienced technical failure.
Major complications occurred in two patients. One patient had undergone PRG after a Billroth II surgery, developed pneumoperitoneum during the PRG procedure, and was later found to have the gastrostomy tube traversing the left hepatic lobe. This patient showed no improvement in abdominal pain and tenderness caused by pneumoperitoneum, despite intravenous antibiotic treatment for more than 4 days, so the tube was removed 8 days after the PRG (Fig. 2). The other patient had undergone a Billroth I surgery, and two days after the PRG procedure, the patient had bleeding from the short gastric artery and was treated with transcatheter embolization using gelatin sponge particles and three microcoils.
Univariable analysis showed that the technical success rate was significantly greater in the patients with distal gastrectomy with gastroduodenostomy (Billroth I procedure) (100%, 6/6) than in the patients with subtotal gastrectomy with gastrojejunostomy (Billroth II procedure) (31%, 4/13) (p = 0.011) (Table 1). However, the technical success was not significantly related to any other factors analyzed (Table 1).
Percutaneous radiologic gastrostomy in the surgically altered stomach presents technical challenges, because the approaches for gastrostomy placement are angled toward the fundus rather than perpendicular to the abdominal wall, in which only the unfavorable puncture windows are available (8, 19). In our study, PRG was initially attempted in 79% (15/19) of all 19 referred patients. The technical success rate was 53% (10/19) for all referred patients and 67% (10/15) for the patients with attempted puncturing.
The causes of technical failure in our study were the high-lying remnant stomach and bowels located anterior to the stomach. The technical feasibility was determined by anteroposterior and lateral radiographs with the patients on the fluoroscopic table. Upper gastrointestinal barium studies were helpful before PRG, in patients with history of gastrectomy, in defining more precisely the anatomic relationship between the stomach and the transverse colon (20). The high-lying stomach positioned under the lower left rib cage was also a common cause of technical failure. We assumed that a relatively larger remnant stomach remained after a distal gastrectomy with gastroduodenostomy (Billroth I gastrectomy) than after a subtotal gastrectomy with gastrojejunostomy (Billroth II gastrectomy), which contributed to the higher technical success in the patients with distal gastrectomy with gastroduodenostomy. We assume that a smaller 21-gauge needle is sharper and thus produces less tissue resistance during the penetration, compared to other thicker needles (3, 5, 6). When the Chiba needle was piercing the remnant stomach, tenting of the gastric wall was clearly seen as when performing PRG in an intact stomach (9).
The PRG procedure time in our study was not much longer than the time needed for the procedure with native stomach, when using a modified Chiba needle technique with a single gastropexy (6.4 vs. 5.4 minutes) (9). Our procedure time seems considerably shorter compared to the reported procedure time of 10-15 minutes, considering the performance of a series of gastrostomy with a single gastropexy using a 17-gauge puncture needle under ultrasonographic and fluoroscopic guidance (8). The shorter procedure times in our study are likely, or at least in part, due to the easier puncturing with a smaller diameter needle and a single gastropexy and using only the fluoroscopic guidance.
In the cases of PRG with technical failure, PRJ can be an alternative option. The PRJ procedure is gradually but slowly gaining acceptance, perhaps because of the technical difficulties in targeting and puncturing mobile and compliant jejunum (21). Hu et al. (18) reported the usefulness of a modified Chiba-needle technique with a single gastropexy for PRJ, and the successful procedures in all of their 51 patients. In our study, PRJ using the same modified Chiba-needle technique resulted in 100% technical success in 9 patients with technical failure of PRG.
The overall incidence of complications after PRG, reported in the available literature, is in the range of 8-30%, with the serious events requiring further treatment occurring in 1-4% of the patients (2, 3, 4, 5, 6, 7, 8, 9). Tube passage through the liver and pneumoperitoneum occurred in only one study patient. Although the tube was removed without any further problems, this complication could have been prevented by using ultrasound to clearly demarcate the left hepatic lobe prior to PRG attempt. Because there is a longer course from the abdominal wall to the anterior wall of the remnant stomach, there is a possibly increased risk of pneumoperitoneum and penetration injury after PRG in patients with a history of partial gastrectomy compared to the patients with intact stomach. Bleeding occurred in another patient in this study, and this patient underwent transcatheter arterial embolization which provided safe and effective conservative management and hemostasis (22). The gastrostomy tube was maintained.
In conclusion, PRG can be successfully performed using the one-anchor technique, as it was successful in approximately half of the post-gastrectomy patients included in the study. The success rate is significantly higher in the patients with Billroth I gastrectomy than in the patients with Billroth II gastrectomy. When there is a technical failure of PRG, the PRJ can be a therapeutic alternative.
Figures and Tables
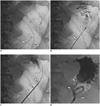 | Fig. 1Successful percutaneous radiologic gastrostomy in patient with distal gastrectomy with gastroduodenostomy.
A. Chiba needle (arrows) is advanced into remnant stomach which was inflated with 250 mL of air through nasogastric tube (arrowheads). Intragastric location of Chiba needle is confirmed with contrast injection. B. Chiba needle is then exchanged for Neff catheter (arrows) using 0.018-inch guide wire. C. Cope suture anchor (arrows in C, D) is deployed into stomach through Neff catheter using 0.035-inch guide wire. Then puncture site is serially dilated using dilator (arrowheads). D. 14-Fr locking-loop catheter (arrowheads) is inserted into remnant stomach. There is good passage of contrast medium without leakage.
|
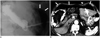 | Fig. 2Pneumoperitoneum developed three days following percutaneous radiologic gastrostomy (PRG).
A. Lateral view, which was obtained three days after PRG, shows pneumoperitoneum (arrows). There was neither pneumoperitoneum nor contrast leakage on one-day follow-up radiograph (not shown). B. CT scan obtained three days after PRG shows large amount of pneumoperitoneum (arrows) as well as passage of gastrostomy tube through left hepatic lobe (arrowheads). Gastrostomy tube was removed with gradual resolution of pneumoperitoneum (not shown).
|
References
1. Deutsch LS, Kannegieter L, Vanson DT, Miller DP, Brandon JC. Simplified percutaneous gastrostomy. Radiology. 1992; 184:181–183.
2. Ho SG, Marchinkow LO, Legiehn GM, Munk PL, Lee MJ. Radiological percutaneous gastrostomy. Clin Radiol. 2001; 56:902–910.
3. Coleman CC, Coons HG, Cope C, Derauf BJ, Krenzel C, Epstein DH, et al. Percutaneous enterostomy with the Cope suture anchor. Radiology. 1990; 174(3 Pt 1):889–891.
4. Given MF, Hanson JJ, Lee MJ. Interventional radiology techniques for provision of enteral feeding. Cardiovasc Intervent Radiol. 2005; 28:692–703.
5. Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005; 56:84–90.
6. Dewald CL, Hiette PO, Sewall LE, Fredenberg PG, Palestrant AM. Percutaneous gastrostomy and gastrojejunostomy with gastropexy: experience in 701 procedures. Radiology. 1999; 211:651–656.
7. Given MF, Lyon SM, Lee MJ. The role of the interventional radiologist in enteral alimentation. Eur Radiol. 2004; 14:38–47.
8. Lorentzen T, Nolsøe CP, Adamsen S. Percutaneous radiologic gastrostomy with a simplified gastropexy technique under ultrasonographic and fluoroscopic guidance: experience in 154 patients. Acta Radiol. 2007; 48:13–19.
9. Shin JH, Song HY, Kim TH, Kim KR, Choi KE, Kim JH. Percutaneous radiologic gastrostomy: a modified Chiba-needle puncture technique with single gastropexy. Abdom Imaging. 2010; 35:189–194.
10. Swetech J, Hasan S. Combined endoscopic and surgical modalities for percutaneous endoscopic gastrostomy placement in the setting of subtotal gastrectomy with a gastric pull up. Gastrointest Endosc. 1990; 36:316–317.
11. Kurchin A, Halloran W, Kornfield R. Assessing the feasibility of percutaneous endoscopic gastrostomy following gastrectomy. Gastrointest Endosc. 1988; 34:366–367.
12. Varney RA, vanSonnenberg E, Casola G, Sukthankar R. Balloon techniques for percutaneous gastrostomy in a patient with partial gastrectomy. Radiology. 1988; 167:69–70.
13. Stellato TA, Gauderer MW, Ponsky JL. Percutaneous endoscopic gastrostomy following previous abdominal surgery. Ann Surg. 1984; 200:46–50.
14. Singh P, Kahn D, Greenberg R, Indaram A, Pooran N, Bank S. Feasibility and safety of percutaneous endoscopic gastrostomy in patients with subtotal gastrectomy. Endoscopy. 2003; 35:311–314.
15. Rosenzweig TB, Palestrant AM, Esplin CA, Gilsdorf RB. A method for radiologic-assisted gastrostomy when percutaneous endoscopic gastrostomy is contraindicated. Am J Surg. 1994; 168:587–590.
16. Perona F, Castellazzi G, De Iuliis A, Rizzo L. Percutaneous radiologic gastrostomy: a 12-year series. Gut Liver. 2010; 4:Suppl 1. S44–S49.
17. Yang ZQ, Shin JH, Song HY, Kwon JH, Kim JW, Kim KR, et al. Fluoroscopically guided percutaneous jejunostomy: outcomes in 25 consecutive patients. Clin Radiol. 2007; 62:1061–1065.
18. Hu HT, Shin JH, Song HY, Kim JH, Yoon HK, Gwon DI, et al. Fluoroscopically guided percutaneous jejunostomy with use of a 21-gauge needle: a prospective study in 51 patients. J Vasc Interv Radiol. 2009; 20:1583–1587.
19. Thornton FJ, Fotheringham T, Alexander M, Hardiman O, McGrath FP, Lee MJ. Amyotrophic lateral sclerosis: enteral nutrition provision--endoscopic or radiologic gastrostomy? Radiology. 2002; 224:713–717.
20. Cantwell CP, Gervais DA, Hahn PF, Mueller PR. Feasibility and safety of infracolic fluoroscopically guided percutaneous radiologic gastrostomy. J Vasc Interv Radiol. 2008; 19:129–132.
21. van Overhagen H, Ludviksson MA, Laméris JS, Zwamborn AW, Tilanus HW, Dees J, et al. US and fluoroscopic-guided percutaneous jejunostomy: experience in 49 patients. J Vasc Interv Radiol. 2000; 11:101–106.
22. Seo N, Shin JH, Ko GY, Yoon HK, Gwon DI, Kim JH, et al. Incidence and management of bleeding complications following percutaneous radiologic gastrostomy. Korean J Radiol. 2012; 13:174–181.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download