Abstract
Objective
To compare the efficacy and adverse effects of endovenous foam sclerotherapy (EFS) and liquid sclerotherapy (ELS) using a microcatheter for the treatment of varicose tributaries.
Materials and Methods
From December 2007 to January 2009, patients with venous reflux in the saphenous vein were enrolled. The foam or liquid sclerosant was injected through a microcatheter just before endovenous laser ablation (EVLA). Patients were evaluated for the technical success, clinical success, and procedure-related complications during the procedure and follow-up visits.
Results
A total of 94 limbs were included: 48 limbs (great saphenous vein [GSV], 35; small saphenous vein [SSV], 13) were managed using EFS and EVLA (foam group; FG), and 46 limbs (GSV, 37; SSV, 9) were treated by ELS and EVLA (liquid group; LG). Varicose tributaries demonstrated complete sclerosis in 92.7% with FG and in 71.8% with LG (p = 0.014). Bruising (78.7% in FG vs. 73.2% in LG, p > 0.05), pain or tenderness (75.6% in FG vs. 51.2% in LG, p = 0.0237) were noted. Hyperpigmentation (51.2% in FG vs. 46.2% in LG, p > 0.05) was found.
Conclusion
Endovenous foam sclerotherapy using a microcatheter is more effective than ELS for eliminating remnant varicose tributaries prior to EVLA. However, EFS is more commonly associated with local complications such as pain or tenderness than ELS. Furthermore, both techniques seem to prolong the duration of hyperpigmentation along with higher costs.
Sclerotherapy is a well-tolerated and highly efficacious treatment for varicose and telangiectatic leg veins (1). Sclerosing solutions act by inducing endothelial damage (endosclerosis), which eventually leads to endofibrosis of the treated vessels. Sclerotherapy is effective when the endothelial damage and the associated vascular necrosis are sufficient to destroy the entire vessel wall (1, 2).
The foam sclerosant, which is made of a mixture of air and a sclerosant such as polidocanol or sodium tetradecyl sulfate, has been introduced in sclerotherapy with the aim of increasing the efficacy and safety of the treatment. The foam might be advantageous over the traditional sclerosants for the following reasons: foam sclerosants are compact solutions that displace the blood column rather than being dissolved in the circulating blood; foam adheres better than traditional sclerosants to the walls of the vein; and foam has high capacity to provoke spasm, and combined with the high adherence, it allows greater contact with the endothelium. In fact, one study suggested that investigations into the treatment of small vein varices revealed that the foam resulted in 20% of more improvement in appearance compared to liquid sclerosants (1).
Several different sclerosing agents and various sclerotherapy techniques have been utilized up until today. Sigg (3) combined sclerotherapy and compression therapy, and started to perform this method on the distal veins. Later, Fegan (4) applied compression sclerotherapy to perforating veins. It has been observed that the application of postsclerosis compression following sclerotherapy increases the effectiveness of the technique (5).
One of the studies used another method of sclerotherapy to treat varicose tributaries. In this method, the contrast media was used to verify the exact orifice of the varicose tributaries which were selected using a microcatheter and guide wire under fluoroscopy. Direct endovenous sclerotherapy using foam was then performed through this microcatheter for the incompetent saphenous vein, before the endovenous laser ablation. This obliterated the varicose tributaries and eliminated the need for the additional percutaneous sclerotherapy or phlebectomy during the follow-up period. According to a previous study (6), the degree of hyperpigmentation and pain or tenderness where the sclerotherapy was performed was not low.
Therefore, the aim of our study was to compare the efficacy and adverse effects of treatment using endovenous foam and liquid sclerotherapy using a microcatheter for treating varicose tributaries.
From December 2007 to January 2009, all patients with varicose veins (CEAP classification: C2 and C3) (Table 1) (7) in unilateral or bilateral lower extremities who visited the out-patient departments of Vascular surgery and Thoracic and cardiovascular surgery were referred to an interventional radiologist and underwent duplex ultrasound examination. Venous reflux was defined as reverse flow in the saphenous vein of > 0.5 second in duration, with release of the calf after compression while standing and after a Valsalva maneuver in the supine position.
We excluded patients younger than 18 years of age and those with nonpalpable pedal pulses, deep vein thrombosis, past history of surgery for varicose vein, inability to ambulate, generally poor health, and those who were pregnant, nursing, or planning to become pregnant at some time during the course of treatment (6). The informed consent was obtained from the patients under the following conditions: the patient had significant venous reflux in the duplex ultrasound with visible varicose tributaries (C2), the patient wanted to remove tributaries, and the patient was applicable to none of the exclusion criteria. This study was approved by the Institutional Review Board.
The patient was brought to the angiography suite and was placed in supine position on the fluoroscopy table. Ultrasound examination and a venogram with contrast media were performed to verify the exact location of the orifice of the varicose tributaries. A 0.035-inch guide wire was then advanced and a 5-Fr catheter (Cordis, Miami, FL, USA) was advanced over the guide wire into the saphenous vein under ultrasound and fluoroscopic guidance. Once the guiding catheter was stabilized and a 2.3-Fr or 2.6-Fr microcatheter was placed into the guiding catheter coaxially, selective catheterization of the varicose tributaries followed by a venogram through the microcatheter was performed, to ascertain the connection to the deep venous system and the exact extent of the varicose tributaries (6).
The foam or liquid sclerosant was injected through the microcatheter (Fig. 1). The foam was produced using Tessari's method, using 1% polidocanol or sodium tetradecyl sulfate mixed with contrast medium (1:2). Two syringes that included sclerosant and air (1:4-5) respectively were attached using a three-way stopcock, and a stable sclerosing foam was obtained by mixing them through multiple passages between the two syringes. After mixing, homogeneous white foam was created, and 2 to 2.5 mL of the foam was aspirated using a 3-mL syringe. The liquid sclerosant was made using 1% polidocanol or sodium tetradecyl sulfate mixed with contrast medium in a 3-mL syringe. Under fluoroscopy, the foam or liquid sclerosant was injected into the tributaries and external compression was exerted for about 5 minutes.
After completion of the endovenous sclerotherapy, the microcatheter was removed and a guiding catheter was advanced. A 600 µm bare-tipped laser fiber of 980-nm wavelength was inserted into the guiding catheter. Using ultrasound guidance, tumescent solution consisting of 100-250 mL of 0.05% lidocaine was delivered along the course of the saphenous vein within the fascial envelope. Endovenous laser ablation (EVLA) was performed with the pull-back method, and linear endovenous energy density of 50 J/cm to 120 J/cm was applied in a continuous mode.
After the procedure, the patient was discharged with a class II full-thigh graduated support stocking to be worn at all times, except during sleeping or showering, for at least one month. Patient was given a prescription for analgesics for 3 to 7 days.
The patients were evaluated for procedure-related complications at the out-patient department at 1 week, and at 1, 3, 6, and 12 months follow-up visits, with the interventional radiologist performing duplex ultrasound at all visits.
The technical success of the endovenous sclerotherapy was defined by the successfully selective catheterization and the adequate injection of the foam into the varicose tributaries (6). The clinical success was considered to have been achieved when the ablated saphenous vein and the varicose tributaries, where the endovenous sclerotherapy was done, were observed to be successfully closed on ultrasound during the follow-up (6). The duplex ultrasound criteria for successful closure included non-compressible veins and absence of blood flow within the entire varicose tributary system and the saphenous vein. We compared the foam and liquid endovenous sclerotherapy groups for the following: the technical success; clinical success rate; and complications such as pain or tenderness, ecchymosis, and hyperpigmentation. The chi-square test and Fisher's exact test were used to make the comparisons and p value < 0.05 was considered significant.
A total of 83 patients and 94 limbs (great saphenous vein [GSV], 72; small saphenous vein [SSV], 22) were included. Among them, 41 patients and 48 limbs (GSV, 35; SSV, 13) were managed using endovenous foam sclerotherapy followed by 980-nm EVLA, and 42 patients and 46 limbs (GSV, 37; SSV, 9) were treated by endovenous liquid sclerotherapy and EVLA. Technical success, in selective catheterization and injection of sclerosant into the varicose tributaries, was seen in all 48 limbs (100%) in the foam endovenous sclerotherapy group (FG), and in all 46 limbs in the liquid endovenous sclerotherapy group (LG).
In FG, the follow-up results at 1 week were obtained in 47 (97.9%) of 48 limbs; and 45 (95.7%) of 47 limbs at 1-week follow-up showed complete closure in the treated saphenous veins. The continued closure of the treated saphenous veins was seen in 39 (95.1%) of 41 limbs at the 1-month follow-up, all 35 limbs (100%) at the 3-month follow-up, all 27 limbs (100%) at the 6-month follow-up, all 12 limbs (100%) at the 1-year follow-up, and all 6 limbs (100%) at the 2-year follow-up. In LG, the follow-up results were obtained in 41 (89.1%) of 46 limbs at the 1-week follow-up; and 40 (97.6%) of 41 limbs at the 1-week follow-up showed complete closure of the treated saphenous vein. The continued closure of the treated saphenous vein in LG was seen in all 39 limbs at the 1-month follow-up, all 33 limbs at the 3-month follow-up, all 19 limbs at the 6-month follow-up, and all 11 limbs at the 1-year follow-up. None of the limbs that showed complete occlusion in the saphenous vein at the 1-week follow-up demonstrated the reappearance of blood flow and compressibility during the later follow-ups. There was no significant difference between the two groups, in the closure rates of the saphenous vein treated by EVLA.
The varicose tributaries treated by endovenous sclerotherapy demonstrated no visible signs of vascularity or compressibility along their entire course in 38 (92.7%) of 41 limbs at the 1-month follow-up in FG. In LG, the complete sclerosis was seen in 28 (71.8%) of 39 limbs at the 1-month follow-up. This difference between FG and LG at the 1-month follow-up was statistically significant (p = 0.014). The patients who did not show complete sclerosis during the follow-up underwent percutaneous sclerotherapy between the 1-month and 3-month follow-ups (Table 2). The volume of foam which was used for sclerotherapy ranged from 1 to 14 mL (mean, 4.52 mL) and the volume of liquid ranged from 2 to 8 mL (mean, 2.96 mL). None of the limbs that had complete sclerosis in the varicose tributaries at the 1-week follow-up showed any blood flow during the further follow-up periods.
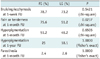
The two frequent complications of bruising and pain or tightness were noted. Bruising was noted in 78.7% of FG and in 73.2% of LG at the 1-week follow-up, but this was asymptomatic and resolved completely in all limbs by the 1-month follow-up. There was no statistically significant difference between the two groups. For the pain or tenderness over the treatment site, there were complaints in about 75.6% of FG and 51.2% of LG at the 1-month follow-up, and this difference was statistically significant (p = 0.0237) (Table 3). These symptoms were greatly improved or resolved in 3 to 6 months. However, 2 out of 12 limbs in FG (16.7%) still had pain in varicose tributaries even at the 1-year follow-up, but none of the 11 patients in LG complained of pain or tenderness at the 1-year follow-up.
The hyperpigmentation was noted in 21 (51.2%) out of 41 limbs in FG and in 18 (46.2%) out of 39 limbs in LG, at the 1-month follow-up. In most of the cases, the symptom improved during the further follow-up periods. However, 3 (25%) out of 12 limbs in FG and 2 (18.1%) out of 11 limbs in LG still had obvious hyperpigmentation at the 1-year follow-up. There was no statistical significance between the two groups (Table 2).
The paresthesia was detected in 1 (2.4%) out of 41 limbs in FG and in 1 (2.6%) out of 39 limbs in LG, at the 1-month follow-up. However, this symptom was relatively mild, well-tolerated, and completely disappeared by the 6-month follow-up. This difference was not statistically significant (Table 2).
One patient (2.1%) in FG noted a transient visual disturbance similar to a flash and a mild headache soon after the sclerotherapy, but none was reported in LG. These symptoms were mild and completely resolved within 30 minutes. There were no significant complications such as skin burn, skin necrosis, pulmonary embolism, cerebral infarction, or allergic reaction.
Compression sclerotherapy has been used for many years in the treatment of varicose veins associated with GSV incompetence. The advantages of compression sclerotherapy include no requirement for anesthesia or hospitalization and the patients returning to normal life soon after the procedure. Indeed, sclerotherapy is relatively a safe and less costly procedure compared to surgery (8, 9, 10). However, there are several drawbacks to sclerotherapy including the risks associated with intra-arterial injection, multiple hospital visits, and the need for multiple needle punctures (11, 12, 13, 14). Therefore, safe injections are to be carried out by the experienced physicians in ultrasonography-guided sclerotherapy, in order to give improved results in the treatment of a refluxing GSV (8, 15).
These methods for sclerotherapy are performed percutaneously and are often used for treating varicose tributaries during the follow-up period, after eliminating the reflux source such as EVLA for GSV or SSV. In a previous investigation, researchers tried to reduce additional percutaneous sclerotherapy for incompetent saphenous vein during the follow-up period after EVLA, and they performed endovenous sclerotherapy using a microcatheter under fluoroscopy and venogram-guidance. They concluded that this method could serve as a way to reduce additional follow-up treatment with percutaneous sclerotherapy after laser ablation (6). Their study used the foam sclerosant which consisted of sodium tetradecyl sulfate and ambient air, and they were able to achieve successful sclerosis in 89% of the varicose tributaries. This means that 89% of the varicose tributaries treated by endovenous foam sclerotherapy did not undergo additional percutaneous sclerotherapy or phlebectomy during the follow-up. However, their study showed 53% of hyperpigmentation in the sites at 1-month follow-up; and it is well-known that hyperpigmentation tends to occur more often with foam sclerotherapy compared to liquid sclerotherapy (16, 17).
Using foam has obvious advantages over liquid sclerosant, which includes the ability of reducing the amount and concentration of the sclerosing liquid needed. The sclerosing foam displaces the blood with very little drug dilution of the blood, and the active surface of the drug is increased by the foam preparation. Ultrasonography-guided foam sclerotherapy provides favorable results, because sclerosing foam is highly echogenic and immediate spasm in the GSV is obtained (8, 18). In the previous articles comparing foam sclerotherapy to liquid sclerotherapy, the foam sclerotherapy showed greater efficacy; and the investigations into the treatment of small vein varices, including telangiectasias, found that the foam results in 20% of the improved appearance relative to the liquid sclerosant (19, 20). Alòs et al. (17) published a randomized controlled trial comparing foam versus liquid sclerotherapy in reticular veins and postsurgical varices with one year of follow-up. The patients served as their own controls, with one vein treated by foam and another vein, either a vein in the same limb or one in the other limb, was treated by liquid sclerotherapy. Polidocanol foam was used and the concentration, which is variable according to the vein diameter, was lowered by half compared to the liquid. At three months, the efficacy rate was 94.4% for the foam versus 53% for the liquid. Therefore, it was concluded that the foam sclerotherapy was more effective than the liquid sclerotherapy. However, we also should take into consideration the local side-effects that were more common with the foam sclerotherapy in that study (17, 20).
In our study, the complete sclerosis rate for varicose tributaries in the endovenous foam sclerotherapy group was higher than in the endovenous liquid sclerotherapy group, and this difference between the groups was statistically significant. Therefore, we can suggest that the endovenous foam sclerotherapy is more effective and is better in reducing the need for additional percutaneous sclerotherapy or phlebectomy to eliminate remnant varicose tributaries, compared to LG.
However, the pain or tenderness was notably more frequent in FG than in LG at the 1-month follow-up, although they greatly improved over the further follow-up period. In addition, although hyperpigmentation tended to be higher in FG than in LG, its incidence was not low even in LG as both 25% of FG and 18.1% of LG patients still showed hyperpigmentation even at the 1-year follow-up. We reasonably assumed that these higher rates of pain or tenderness and hyperpigmentation, relative to other reports in terms of the 1-year follow-up, could be the results of employing different types of sclerosant and technique of endovenous slcerotherapy. In endovenous sclerotherapy, a microcatheter is used and an operator is needed to push the sclerosant strongly to deliver the foam or liquid sclerosant into the varicose tributaries. Therefore, a strong injection could locally dilate the tributaries and cause some pain. In addition, the appropriate compression was not able to be achieved in the tributaries after the successful endovenous foam sclerotherapy, because the operator needed to perform EVLA immediately after the sclerotherapy. Therefore, we suggest that this could have resulted in the higher rate and persistence of the hyperpigmentation.
Rabe et al. (16) has reported the outcome of another randomized controlled trial comparing the liquid and foam sclerotherapy. In this study, the saphenous trunks were injected with either 4 mL of 3% polidocanol liquid or 5 mL of 3% polidocanol foam. This achieved obliteration of the saphenous vein at three months in the 69% of the foam group compared to 27% of the liquid group. This author commented that the amount of foam used was probably a rather small volume for a fairly large saphenous trunk. Contrary to that study, the endovenous foam sclerotherapy in our patients needed a greater amount of sclerosant than in liquid sclerotherpy. We reasonably assumed that this is due to the use of fluoroscopy for guidance. Under the fluoroscopy, we defined a successful injection of sclerosant into the varicose tributaries as a good distribution and mixing of the sclerosant with the contrast media, along the entire course of the varicose tributaries. In fact, the liquid sclerosant has more radioopacity than the foam sclerosant under fluoroscopy, because the foam contains air that reduces the radioopacity when the mixing with the contrast media. Therefore, more scelrosant was injected in foam sclerotherapy than in liquid sclerosant, in order to obtain good distribution of the foam under fluoroscopy for complete achievement of sclerosis in the varicose tributaries.
Transient visual disturbance and mild headache occurred only in FG. However, these symptoms disappeared within 30 minutes and their incidence was extremely low.
This study has some limitations. First, the study was retrospective. Second, there was a risk of exposure to radiation and contrast media from the venography and fluoroscopy, which were used for the guidance of procedures. Third, the cost of the procedure was high due to the use of contrast media and a microcatheter.
In conclusion, despite these limitations, the endovenous foam sclerotherapy using a microcatheter is more effective than the endovenous liquid sclerotherapy, for eliminating remnant varicose tributaries after EVLA. However, endovenous foam sclerotherapy is more commonly associated with local complications, such as pain or tenderness, than endovenous liquid sclerotherapy. Furthermore, both techniques seemed to make the hyperpigmentation last longer and have higher costs.
Figures and Tables
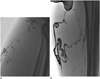
Fig. 1
Fluoroscopic images after injection of foam (A) or liquid (B) sclerosant.
Sclerosing foam (A) or liquid sclerosant (B) mixed with contrast media were injected into varicose tributaries using microcatheter (double arrows) under fluoroscopic guidance. Air bubbles can be seen clearly in foam sclerotherapy (arrows).
References
1. Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatol Surg. 2005; 31:631–635. discussion 635.
2. Goldman MP, Bergan JJ. Sclerotherapy: treatment of varicose and telangiectatic leg veins. 3rd ed. St. Louis: Mosby;2001. p. 1–6.
3. Sigg K. The treatment of varicosities and accompanying complications; (the ambulatory treatment of phlebitis with compression bandage). Angiology. 1952; 3:355–379.
4. Fegan WG. Continuous compression technique of injecting varicose veins. Lancet. 1963; 2:109–112.
5. Uncu H. Sclerotherapy: a study comparing polidocanol in foam and liquid form. Phlebology. 2010; 25:44–49.
6. Park SW, Yun IJ, Hwang JJ, Lee SA, Kim JS, Chang SH, et al. Fluoroscopy-guided endovenous foam sclerotherapy using a microcatheter in varicose tributaries followed by endovenous laser treatment of incompetent saphenous veins: technical feasibility and early results. Dermatol Surg. 2009; 35:804–812.
7. Hardman RL, Rochon PJ. Role of interventional radiologists in the management of lower extremity venous insufficiency. Semin Intervent Radiol. 2013; 30:388–393.
8. Yamaki T, Nozaki M, Iwasaka S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg. 2004; 30:718–722. discussion 722.
9. Biemans AA, Kockaert M, Akkersdijk GP, van den Bos RR, de Maeseneer MG, Cuypers P, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. J Vasc Surg. 2013; 58:727–734.
10. Hamdan A. Management of varicose veins and venous insufficiency. JAMA. 2012; 308:2612–2621.
11. Barrett JM, Allen B, Ockelford A, Goldman MP. Microfoam ultrasound-guided sclerotherapy treatment for varicose veins in a subgroup with diameters at the junction of 10 mm or greater compared with a subgroup of less than 10 mm. Dermatol Surg. 2004; 30:1386–1390.
12. Zimmet SE. Sclerotherapy treatment of telangiectasias and varicose veins. Tech Vasc Interv Radiol. 2003; 6:116–120.
13. Breu FX, Guggenbichler S. European Consensus Meeting on Foam Sclerotherapy, April, 4-6, 2003, Tegernsee, Germany. Dermatol Surg. 2004; 30:709–717. discussion 717.
14. Breu FX, Guggenbichler S, Wollmann JC. Second European Consensus Meeting on Foam Sclerotherapy. Duplex ultrasound and efficacy criteria in foam sclerotherapy from the 2nd European Consensus Meeting on Foam Sclerotherapy 2006, Tegernsee, Germany. Vasa. 2008; 37:90–95.
15. Schadeck M. Ultrasound guided sclerotherapy. In : Schadeck M, editor. Duplex and Phlebology. Napoli: Gnocchi;1994. p. 115–128.
16. Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg. 2008; 35:238–245.
17. Alòs J, Carreño P, López JA, Estadella B, Serra-Prat M, Marinel-Lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. Eur J Vasc Endovasc Surg. 2006; 31:101–107.
18. Cavezzi A, Frullini A, Ricci S, Tessari L. Treatment of varicose veins by foam sclerotherapy: two clinical series. Phlebology. 2002; 17:13–18.
19. Hamel-Desnos C, Allaert FA. Liquid versus foam sclerotherapy. Phlebology. 2009; 24:240–246.
20. Coleridge Smith P. Saphenous ablation: sclerosant or sclerofoam? Semin Vasc Surg. 2005; 18:19–24.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download