Abstract
Objective
To evaluate the safety and efficacy of transcatheter arterial chemoembolization (TACE) in patients with infiltrative hepatocellular carcinoma (HCC) and to identify the prognostic factors associated with patient survival.
Materials and Methods
Fifty two patients who underwent TACE for infiltrative HCC were evaluated between 2007 and 2010. The maximum diameter of the tumors ranged from 7 cm to 22 cm (median 15 cm). Of 46 infiltrative HCC patients with portal vein tumor thrombosis, 32 patients received adjuvant radiation therapy for portal vein tumor thrombosis after TACE.
Results
The tumor response by European Association for the Study of the Liver criteria was partial in 18%, stable in 47%, and progressive in 35% of the patients. The median survival time was 5.7 months (Kaplan-Meier analysis). The survival rates were 48% at six months, 25% at one year, and 12% at two years. In the multivariable Cox regression analysis, Child-Pugh class (p = 0.02), adjuvant radiotherapy (p = 0.003) and tumor response after TACE (p = 0.004) were significant factors associated with patient survival. Major complications occurred in nine patients. The major complication rate was significantly higher in patients with Child-Pugh B than in patients with Child-Pugh A (p = 0.049, χ2 test).
Conclusion
Transcatheter arterial chemoembolization can be a safe treatment option in infiltrative HCC patients with Child Pugh class A. Child Pugh class A, radiotherapy for portal vein tumor thrombosis after TACE and tumor response are good prognostic factors for an increased survival after TACE in patients with infiltrative HCCs.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide with an increasing incidence in industrialized countries (1). Extensive ultrasound screening programs have helped to detect HCCs at an early stage during which they are amenable to various treatment options such as hepatic resection, percutaneous ethanol injection and thermoablation. Although these curative treatment options are known to improve survival, they can only be performed in 30% of patients with HCCs (2).
Treatment of infiltrative HCCs (i.e., with indistinct borders and a lack of typical enhancement pattern) is challenging for following reasons: 1) most infiltrative HCC patients present with advanced disease as infiltrative HCC may be difficult to diagnose given that it commonly lacks a well-demarcated boundary on cross-sectional imaging and can thus blend into the background of the cirrhotic liver (3, 4), 2) it is difficult to evaluate the tumor extent within the liver due to the poorly delineated tumor boundary, and 3) because of its large, diffuse nature and propensity to involve the portal vein, diffuse infiltrative HCC may be difficult to treat and the treatment may increase the risk of serious complications (5). However, treatment options for patients with diffuse, infiltrative HCCs are limited and data are insufficient regarding the presentation, treatment and outcomes of patients with infiltrative HCCs (5).
In recent years, transcatheter arterial chemoembolization (TACE) has been considered for certain patients with unresectable HCC to be used for palliative purposes (6). Previous studies have clearly shown that TACE could safely prolong the survival in selected patients with unresectable HCC and with or without portal vein invasion (7, 8, 9, 10, 11, 12, 13). In this study, we evaluated the safety and efficacy of TACE in patients with infiltrative HCC and attempted to identify the prognostic factors associated with patient survival after TACE for infiltrative HCC.
This retrospective study was approved by our Institutional Review Board. The medical and imaging records of the enrolled patients were thoroughly reviewed. Indications of TACE for HCC at the authors' institution are 1) unresectable HCC because of either advanced stage or insufficient hepatic reserve and 2) tumors that are unsuitable for other local treatments such as radiofrequency ablation or percutaneous ethanol injection because of tumor size, presence of multiple lesions (i.e., more than three), vascular invasion or subcapsular lesions. Exclusion criteria for TACE were any contraindication to the use of an arterial procedure, such as impaired clotting (platelet count < 50000/mm3 or prothrombin activity < 50%), bacterial infection or renal failure. Between January 2007 and January 2010, a total of 52 patients with infiltrative HCCs underwent TACE at our institution. There were 43 men and nine women ranging in age from 39-73 years (mean 56 ± 9 years). Most of these patients (82%) had underlying liver cirrhosis which had been diagnosed on imaging. The hepatitis B surface antigen was positive in 48 patients. The criteria for the diagnosis of HCC were based on the guidelines of the American Association for the Study of Liver Diseases (14). The number of patients in class A was 22 and in class B 30, referring to the degree of liver function according to the Child-Pugh class.
The diameter of the tumors ranged from 7-22 cm (median 15 cm [interquartile range 12-17 cm]) in maximum dimension before the initial treatment. Portal vein tumor thrombosis (main [n = 26], lobar or segmental [n = 20]) was observed in 46 patients (88%). According to the American Joint Committee on Cancer staging system (15), the number of patients in stages IIIA, IIIB, IIIC, IVA and IVB were three, 38, two, three, and six, respectively.
Superior mesenteric and common hepatic angiographies were performed to assess the vascular anatomy, tumor vascularity, tumor extent and portal vein patency. Following the selective cannulation of the right lobar, left lobar or proper hepatic artery using a microcatheter, 0.5 mg/mL of cisplatin dissolved in distilled water, was infused into the hepatic artery for 15 minutes according to the tumor location. The infused dose of cisplatin was 2 mg/kg (4 mL/kg) per patient's weight. Before the 15-minute cisplatin infusion, some cisplatin was set aside to be mixed at a 1:1 ratio in an emulsion of iodized oil (Lipiodol, LaboratoireGuerbet, Cedex, France). It was infused into the segmental feeding artery after the cisplatin infusion (16). The embolization of the feeding arteries was performed by using 1-mm-diameter, absorbable gelfoam slurry (Gelfoam; Upjohn, Kalamazoo, MI, USA) until an arterial flow stasis was achieved. The 1 mm-diameter gelfoam slurry was manually made by cutting a 70 × 50 × 10 mm gelfoam sponge. The aforementioned TACE technique was applied to all patients included in this study. Less embolization with gelfoam slurry was performed in patients with extensive portal vein tumor thrombosis (i.e., main portal vein level) or poor hepatic reserve (Child-Pugh score B).
A radiotherapy focused on portal vein tumor thrombosis was planned after the identification of portal vein tumor thrombosis at the initial presentation or on follow-up imaging after TACE and was started 0.5-4 months (mean 1.03 ± 0.64 months) after initial TACE for HCC with portal vein tumor thrombosis. Eligibility criteria for this radiotherapy were as follows: tumor thrombosis in the main, lobar or segmental portal vein, liver function of Child A or B, an Eastern Co-operative Oncology Group performance status of 0-2, no history of liver radiotherapy, tolerated initial TACE session and signed informed consent (11, 17). The presence of lymph nodes or distant metastases was not part of the exclusion criteria. An adjuvant radiation therapy was not performed in some patients who met those criteria, based on the attending physician's preferences (11). A three-dimensional conformal radiotherapy was used to determine target volumes, radiation ports and dose prescriptions by using a 3-dimensional radiotherapy planning system (Eclipse; Varian, Palo Alto, CA, USA). The gross tumor volume included the portal vein tumor thrombosis and a 2- to 3-cm margin into the contiguous HCC. The dose per fraction to the planning target volume was 2 to 5 Gy at 5 fractions per week. The total dose was determined by the volume of the normal liver and liver function and the maximum dose to the stomach or duodenum. A general guideline was that the fraction of the normal liver treated with more than 50% of the prescribed dose should be less than 50% of the normal liver volume (V50% < 50%) (17).
A follow-up CT was performed one month after each TACE session in order to assess the tumor response to treatment and to allow a timely decision regarding subsequent treatment. The tumor extent was evaluated by measuring the diameter of the heterogeneous or homogeneous enhancing areas on arterial phase images, which corresponded to the washout on the portal or to a delayed phase on dynamic CT or MRI. The tumor response was as previously described, classified into four grades; complete response, partial response, stable disease and progressive disease, according to the European Association for the Study of the Liver criteria (5, 18, 19). A repeat TACE was indicated when there were new tumors, when there was tumor growth or when a residual tumor was detected. The treatment was terminated if the patient could not tolerate the procedure because of a decline in his/her clinical status.
The tumor response was evaluated 1 month after the initial TACE and was categorized as regression including complete response or partial response or non-regression including stable or progressive disease. Mortality resulting from TACE was defined as death within 30 days after TACE. According to the Society of Interventional Radiology reporting standards (20), major complications were defined as any event that resulted in the need for additional treatment, including an increased level of care, hospital stay beyond the observation status and including readmission after initial discharge, permanent adverse sequelae including substantial morbidity and disability, and death. All other complications were classified as minor. The overall patient survival was measured in months from the time of the first chemoembolization.
We evaluated the following prognostic factors for patient survival: age; sex; tumor size; Child-Pugh class; α-fetoprotein (AFP) level; portal vein tumor thrombosis; lymph node or distant metastasis; adjuvant radiotherapy for portal vein tumor thrombosis and tumor response. The tumor sizes and serum AFP levels were classified in two groups on basis of the median values.
Statistical analysis was conducted using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA). We used χ2 test to determine whether there was any difference in the major complications rate according to the Child-Pugh classes. The cumulative survival curves were created using the Kaplan-Meier method and were compared with the results of the log-rank test. Univariable and multivariable Cox regression analysis was performed to assess the factors associated with patient survival. We included significant factors or factors that showed a trend toward statistical significance in a multivariable model (p < 0.1) in the univariable analysis.
The median number of treatment sessions was two per patient (range 1-17 sessions). A postembolization syndrome was developed in 19 of 52 study patients (37%), although it resolved within seven days without any treatment. Chemoembolization-related major complications occurred in nine (17%) study patients. The major complication rate was significantly higher in patients with Child-Pugh B (25.8%, 8/31) than in patients with Child-Pugh A (4.7%, 1/21) (p = 0.049). An acute renal failure occurred in four patients, although these patients eventually recovered after medical treatment. A hepatic failure manifested by encephalopathy or ascites or both occurred in four patients. A hepatic abscess occurred in one patient and was successfully treated by percutaneous drainage and antibiotic therapy. One patient with Child-Pugh class B died within 30 days after the time of chemoembolization because of a post-chemoembolization-related hepatic failure. Therefore was the study patient mortality rate 2% (1 of 52).
A tumor response evaluation after TACE was possible in 49 patients (94%, 49/52) because 1 month follow-up CT evaluation was not performed in three patients due to death within 1 month after TACE (n = 1) or an elevated creatinine level (> 2.0 mg/dL) (n = 2). After TACE, no patient showed complete response, nine patients (18%, 9/49) showed partial response (Fig. 1), 23 patients showed stable disease (47%, 23/49) and 17 (35%, 17/49) patients showed progressive disease despite the therapy. An objective tumor regression (≥ partial response) was achieved in nine patients (18%, 9/49) after TACE.
Thirty-two of the 52 study patients (62%) were treated with an adjuvant radiation therapy for portal vein tumor thrombosis after TACE. Fifty patients died and two remained alive during the follow-up period (mean 10.1 months) with a median survival period of 5.7 months. The patient survival rates were 48% at six months, 25% at one year and 12% at two years after chemoembolization (Fig. 2).
The following variables of p < 0.1, as seen in univariable analysis (Table 1), were entered into the multivariable Cox regression model: Child-Pugh class; tumor response and adjuvant radiation therapy after TACE. Multivariable analysis also confirmed that the Child-Pugh class (p = 0.02), adjuvant radiation therapy after TACE (p = 0.003) and tumor response after TACE (p = 0.004) were significant factors associated with patient survival after TACE (Table 2). The Kaplan-Meier curves determined with these three factors are shown in Figure 3.
Infiltrative HCC is defined as a subtype of liver cancer that presents as an ill-defined, diffuse lesion. It accounts for 7 to 13% of HCCs (4, 21). As infiltrative HCC almost always manifests as an extensive, diffuse tumor and there are only few treatment options other than TACE. TACE is regarded as a strong relative contraindication for infiltrative HCC due to concerns regarding hepatic failure and the grave prognosis caused by a poor hepatic reserve (22). For this reason, although TACE is generally accepted as the only available treatment option, its efficacy for the treatment of infiltrative HCC has rarely been studied. However, Kneuertz et al. (5) and Jang et al. (23) have recently reported that TACE is both safe and feasible in selected patients with infiltrative HCC.
In our study, the median survival of the 52 study patients was 5.7 months. The one-year survival rate was 25% and the two-year survival rate was 12%. The results were quite comparable to those reported by Jang et al. (23), i.e., a median survival of 5.4 months, a survival rate of 29.4% at one year and a survival rate of 15.9% at two years. Approximately 60% of patients showed partial or stable disease after TACE. There was only one (2%) patient who died within 30 days of TACE and this was very similar to the peri-procedural mortality rate of 2.1% reported by Kneuertz et al. (5).
Our multivariable analysis showed that tumor response (partial response vs. stable disease, odds ratio [OR] 1.869, stable disease vs. progressive disease, OR 4.991, and p = 0.004), hepatic reserve (Child-Pugh class B, OR 2.138, p = 0.02), and adjuvant radiation therapy after TACE (OR 0.358, p = 0.003) were significant independent prognostic factors for patient survival. We discovered that patients with either partial or stable disease had a better survival rate than those with progressive disease. As in some other recent reports (23), our study suggested that a poor hepatic reserve was associated with poor patient survival.
An adjuvant radiotherapy was considered once a portal vein tumor thrombosis was detected on initial or follow-up imaging. The rationale for the combined therapy was that a portal vein tumor thrombosis-focused adjuvant radiotherapy performed in patients with advanced HCC may decrease intravascular tumor growth and maintain portal flow, thus preserving liver function, inhibiting intrahepatic tumor spread and thus allowing additional TACE (24, 25, 26). Yoon et al. (17) found that adjuvant radiation therapy combined with TACE offers a safe and feasible treatment option for advanced HCC with portal vein tumor thrombosis. In their report (17), the median survival period after combining TACE and radiotherapy for 412 HCC patients with portal vein tumor thrombosis was 10.6 months. In our series, a total of 32 patients (62%) underwent adjuvant radiotherapy and these patients showed a better survival rate (median survival period of eight months) than those who did not undergo an adjuvant radiotherapy (2.2 months). Therefore, we suggest that this combined approach provides an increased survival benefit for patients with infiltrative HCC, given that the majority of these patients may have combined portal vein tumor thrombosis for which radiotherapy may be an effective treatment (17).
When a physician treats patients with infiltrative HCCs, concerns regarding severe post-procedural complications arising from extensive tumor necrosis, may be an obstacle to perform an aggressive treatment (22). Indeed, TACE-related major complications developed in nine patients (17%) in our study. This was more than the 3% seen in a previous study reporting the results of TACE in 362 patients with HCCs (6). The complication rate in our study was comparable to the 12.5% seen in a previous study (23) reporting the results of TACE in patients with infiltrative HCC. However, in our study occurred the majority of TACE-related complications in patients with poor hepatic reserve and the major complications rate was significantly higher in patients with Child-Pugh B (25.8%) than in patients with Child-Pugh A (4.7%). Therefore, we believe that TACE can be safely performed as the initial treatment in patients with infiltrative HCC but with good hepatic reserve.
Previous studies (6, 27) found that the initial tumor size was a significant predictor of patient survival after TACE. However, the tumor size was not a significant factor for patient survival on multivariable analysis in our study. This might have resulted from differences in the demographics of patients (different composition of tumor types, tumor size). For instance, the majority of our study patients had a large tumor burden (range, 7-22 cm; median 15 cm [interquartile range 12-17 cm]). In addition, a previous study reporting the survival outcomes of TACE for infiltrative HCCs also found tumor size (tumor extent in their study) not as a significant factor on multivariable analysis (23).
The limitations of our study are the inclusion of patients treated at a single medical center only, the absence of a patient control group and the retrospective nature of the study. There are further prospective randomized studies required to evaluate the safety and efficacy of TACE for treating infiltrative HCC. In addition, a radiation therapy was started within one month after TACE in 15 patients among the 32 patients who underwent adjuvant radiation therapy for portal vein tumor thrombosis. Because tumor response was evaluated one month after TACE, this may have influenced the results of tumor response, although the radiation therapy focused on the portal vein tumor thrombosis, not on the primary HCCs.
In conclusion, TACE may be well-tolerated and can be a safe treatment option in patients with infiltrative HCC with Child Pugh class A. A disease of Child-Pugh class A, an adjuvant radiation therapy for portal vein tumor thrombosis after TACE and the tumor response are good prognostic factors for an increased patient survival after TACE in patients with infiltrative HCCs.
Figures and Tables
Fig. 1
Images of 62-year-old patient with HCC.
A. Contrast-enhanced axial CT image in arterial phase showing diffuse infiltrative HCC (arrowheads) with right and main portal vein thrombosis (arrow). B. Hepatic angiographic image showing diffuse tumor staining in right lobe. C. Contrast-enhanced axial CT image in arterial phase obtained 1 month after initial chemoembolization shows lipiodol uptake in tumor and decreased extent of infiltrative HCC (arrowheads). D. Contrast-enhanced axial CT image in arterial phase after additional chemoembolizations (6 months after initial chemoembolization) shows further decreased extent of infiltrative HCC (arrowheads). HCC = hepatocellular carcinoma
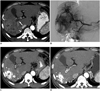
Fig. 2
Kaplan-Meier curve shows overall cumulative survival rates in all 52 patients with infiltrative hepatocellular carcinomas.

Fig. 3
Kaplan-Meier analysis for factors associated with patient survival.
A. Kaplan-Meier curves show patient survival rates according to Child-Pugh classification. Median survival period was 10.0 months for patients with Child-Pugh class disease and 3.4 months for patients with Child-Pugh class B disease (p = 0.001). B. Kaplan-Meier curves show patient survival rates according to adjuvant radiation therapy performed after TACE. Median survival period was 8.0 months for patients who underwent adjuvant radiation therapy after TACE and 2.2 months for patients who did not undergo adjuvant radiation therapy after TACE (p < 0.001). C. Kaplan-Meier curves show patient survival rates according to tumor response. Median survival period was 9.7 months for patients with partial response, 7.3 months for patients with stable disease and 2.7 months for patients with progressive disease (p < 0.001). TACE = transcatheter arterial chemoembolization

References
1. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999; 340:745–750.
2. Takayasu K. Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol. 2012; 42:247–255.
3. Demirjian A, Peng P, Geschwind JF, Cosgrove D, Schutz J, Kamel IR, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011; 15:2089–2097.
4. Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003; 18:189–195.
5. Kneuertz PJ, Demirjian A, Firoozmand A, Corona-Villalobos C, Bhagat N, Herman J, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012; 19:2897–2907.
6. Hu HT, Kim JH, Lee LS, Kim KA, Ko GY, Yoon HK, et al. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol. 2011; 22:917–923.
7. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.
8. Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011; 18:413–420.
9. Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013; 13:60.
10. Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995; 165:315–321.
11. Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009; 24:806–814.
12. Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011; 258:627–634.
13. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002; 35:1164–1171.
14. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
15. Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002; 87:13–15.
16. Yoon HM, Kim JH, Kim EJ, Gwon DI, Ko GY, Ko HK. Modified cisplatin-based transcatheter arterial chemoembolization for large hepatocellular carcinoma: multivariate analysis of predictive factors for tumor response and survival in a 163-patient cohort. J Vasc Interv Radiol. 2013; 24:1639–1646.
17. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012; 82:2004–2011.
18. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
19. Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009; 250:466–473.
20. Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S425–S434.
21. Trevisani F, Caraceni P, Bernardi M, D'Intino PE, Arienti V, Amorati P, et al. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer. 1993; 72:1557–1563.
22. Lopez RR Jr, Pan SH, Hoffman AL, Ramirez C, Rojter SE, Ramos H, et al. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg. 2002; 137:653–657. discussion 657-658.
23. Jang ES, Yoon JH, Chung JW, Cho EJ, Yu SJ, Lee JH, et al. Survival of infiltrative hepatocellular carcinoma patients with preserved hepatic function after treatment with transarterial chemoembolization. J Cancer Res Clin Oncol. 2013; 139:635–643.
24. Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003; 57:113–119.
25. Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005; 61:432–443.
26. Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, et al. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002; 25:189–193.
27. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006; 131:461–469.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download