Abstract
Central venous catheters are the most frequent causes of benign central vein stenosis. We report the case of a 79-year-old woman on hemodialysis through a twin catheter in the right internal jugular vein, presenting with superior vena cava (SVC) syndrome with patent SVC. The clinically driven endovascular therapy was conducted to treat the venous syndrome with a unilateral left brachiocephalic stent-graft without manipulation of the well-functioning catheter. The follow-up was uneventful until death 94 months later.
Central venous catheters (CVCs) are the most frequent causes of benign central vein stenosis, a potentially fatal complication for dialysis patients because they limit the efficiency and duration of native or prosthetic peripheral access routes and can cause malfunctioning of dialysis catheters and reduce the number of veins suitable for catheterization (1). The consequences of venous obstruction depend on the rapidity of development, site of obstruction, presence of effective collateral channels and the presence of a functioning arterio-venous fistula/graft (AVF/AVG) upstream from the obstruction. The stenotic obstruction of one of the brachiocephalic veins may remain completely asymptomatic, if blood flow is adequately compensated by the contralateral vein. On the other hand, a superior vena cava syndrome (SVCS) will appear with edema in the face, neck and trunk if a CVC placed in the contralateral brachiocephalic vein gives rise to thrombosis and occlusion. In this situation, the catheter may continue to work well within the stenotic vein especially if the distal ends of the lumens are positioned within the right atrium and are thus not affected by the fibrin sleeve. We report a case of SVCS due to stenosis of both brachiocephalic veins with a non-infected working dialysis twin catheter in place in the right internal jugular vein (RIJV). Clinical symptoms resolved after the deployment of a stent-graft in the stenotic left brachiocephalic vein (LBCV) without manipulating the catheter. Venous drainage was restored and follow-up was uneventful until the death of the patient of unrelated causes 94 months later.
A 79-year-old female patient presented in 2004 with edema of the arms, neck and face and initial difficulty in breathing; she was on regular dialysis treatment trough a working RIJV CVC. The tunnelled twin catheter (Gemini, Belco, Mirandola, Italy) was placed with both distal ends in the right atrium 27 months before (June 2001). The patient was diagnosed with a CVC-related SVCS. She had an autosomal dominant polycystic kidney disease and underwent dialysis through a left AVF since October 1999. Her medical history included a carcinoma in the left breast with left quadrantectomy and axillary lymph nodes exeresis followed by radiation and hormone therapy. The patient had had several prior left and right AVFs as well as a CVC in the left subclavian vein for several months.
The peripheral veins on both arms were unsuitable for venography due to the previous AVFs. A written informed consent was obtained to perform the endovascular procedure. A digital catheterography was carried out through the working twin catheter (flow max > 400 mL/min) and showed a regular flow in the superior vena cava (SVC). A blocked flow was shown in the right jugular extending to the right brachiocephalic vein (RBCV) after contrast medium injection through the ultrasound-guided RIJV access, with a filling defect in the subclavian vein visible in the late phase referred to thrombosis (Fig. 1A, B). A preserved venous flow was shown despite an old hyperechogenic mural thrombus detected on preliminary sonography and critical stenosis of the LBCV at its opening into the SVC with a left internal jugular vein (LIJV) venography through a 5-Fr valved sheath (Pinnacle Introducer sheath, Terumo, Tokyo, Japan) (Fig. 1C). An 8-Fr introducer was positioned in the right femoral vein and a 40° angled shaped 8-Fr guiding catheter easily advanced into the SVC. Several unsuccessful attempts were made to pass coaxial systems in the occluded RBCV alongside the twin catheter. Eventually, a 0.018-inch guide wire was advanced from the LIJV to pass the LBCV stenosis in road-map modality. A percutaneous transluminal angioplasty (PTA) of the stenosis performed with an 8 mm × 4 cm PTA catheter (Fox, Abbott, Santa Clara, CA, USA) was followed by immediate and complete elastic recoil (Fig. 1D).
A further dilation was judged as useless and thus a stent placement was mandatory; a stent-graft was considered more suitable than a bare metal stent (BMS), due to the potential risk of damaging the polyurethane catheter lines by the uncovered edges of the latter. A 12 mm Viatorr stent-graft (Gore, Flagstaff, AZ, USA) was chosen in order to avoid bridging the opening of the patent LIJV. The 2 cm bare nitinol leading end permitted the stabilization of the stent in front of distal LIJV and the 12 mm caliber was still compatible with a 10-Fr introducer. The catheter introduced through the femoral vein was advanced into the LBCV. The guide wire and catheter introduced through the LIJV were pulled out, leaving the introducer in place. A 65 cm long 10-Fr armed introducer (Super Arrow-Flex, Reading, PA, USA) was positioned in the LBCV over a stiff 300 cm long 0.035-inch guide wire (Spartacore, Abbott, Santa Clara, CA, USA). The narrow valve of the Arrow sheath was manually removed with a forceps allowing the insertion of a 10 cm long, 12 mm Viatorr expanded polytetrafluoroethylene (ePTFE) stent-graft. The stent-graft was released in the LBCV after accurate checking of the correspondence of the leading bare end with the opening of the LIJV by means of contrast injection from the LIJV introducer (Fig. 1E, F). Post dilation was carried out to 12 mm. The subsequent venography showed an excellent flow (Fig. 1G, H). Finally, the introducers were removed and manual compression was carried out.
The signs and symptoms of SVCS regressed and disappeared over the next few days. The catheter worked well and there was no recurrence of SVCS until the patient's death of unrelated causes (complications following a hip fracture) 94 months later in November 2012. A CT scan with contrast medium showed the patency of the stent graft 60 months after the stent graft placement. The CT was carried out to stage the cancer of patient in October 2009 (Fig. 1I, J).
Central vein stenosis (CVS) is a frequent complication of CVCs in dialysis patients. A prevalence of 19-41% of CVS has been reported in hemodialysis patients with previous CVCs. However, a similar incidence of 7-40% has also been reported for first time CVC patients. The causes are high vascular wall shear stress with presence of AVF or AVG and vascular compression or distortion, frequent in aging (1).
It is thought that about 50% of central vein stenosis remains asymptomatic with the most frequent clinical manifestations, due to upstream local hypertension, being massive edema in the arm, the face and any other structure perfused via the SVC. Visible superficial collateral channels and respiratory and neurological symptom are also typical of SVCS. SVCS develops due to a stenosis of the SVC or due to a stenosis of both brachiocephalic veins, if it develops in spite of a patent vena cava (2).
Therapy is either surgical or endovascular. A surgical treatment bears all the risks of a major operation and is unlikely to be safe in dialysis patients with generally several comorbidities. The first-line therapeutic option is represented by endovascular treatment which can be carried out by angioplasty alone or include the positioning of a stent (BMS or covered stent). The NKF-KDOQI guidelines recommend to place a stent in cases of immediate elastic recoil (10-30%, typical in central veins), residual stenosis > 30% and recurrence of stenosis within three months (3). Studies comparing vessel patency after PTA alone and after the positioning of a BMS found no significant difference between both with a primary patency estimated between 12 and 60% equally for both techniques after 12 months (1).
Stent-grafts, used more recently, have shown promising medium and long term results in retrospective single-centre studies in terms of target vessel (39-100% primary patency and 80-100% cumulative patency at 9-12 months) and access circuit patencies (85-94% primary cumulative patency at 12 months) (4, 5, 6, 7).
Superior vena cava syndrome is generally associated with the malfunctioning of long term or tunnelled dialysis catheters, but it can also occur in a well-functioning CVC, as in our case.
In the past, at least one of the two lumens of the catheter was left proximally to the cavoatrial junction to avoid flow recirculation, so CVS could affect at least one of the branches or lumens of the catheter. At present, the favoured approach is to place both lumens of long term catheters within the right atrium to permit high flow during dialysis. This means the situation can arise where a catheter with tips correctly positioned in the right atrium can be the cause of obstruction in one or more of the blood vessels it passes through, while continuing to function perfectly.
In our case, the right transjugular catheter was working and free of infection, but was the cause of SVCS due to a progressive occlusion of the right jugular-brachiocephalic side coincident with the so far asymptomatic pre-occlusive stenosis of the LBCV, the site of a previous catheter.
Our approach was to treat the CVS contralateral to the working, uninfected catheter. The treatment of a CVC-related central vein stenosis without removal of the CVC has been reported before. The technique only seems applicable to very flexible, small caliber CVCs, since although the extravascular tract of the CVC can be left in situ, the intravascular part needs to be repositioned from another endovascular approach before and after stenting of the underlying stenosis (8, 9). It seems unlikely that this maneuver could be accomplished with a large bore twin dialysis catheter with tips positioned in the right atrium as in our case.
A replacement of the CVC was ruled out at the time of treatment because it was judged that this conservative treatment would avoid unnecessary complications: with a contralateral stent-graft in place, the substitution of the CVC could easily be carried out in the angiography theatre if necessary. An over the wire replacement of the right twin CVC after PTA alone or a BMS-assisted PTA would have been mandatory if it had not been possible to re-canalize the left venous axis or if there had been a CVC infection, as stent-grafts are contraindicated where there is an infection. In the last instance, the right common femoral vein approach would have allowed to perform the pull-through technique.
Another point deserving some explication in our case is the choice of stent-graft. We report the off label use of a Viatorr 12 mm ePTFE stent-graft (Gore, Flagstaff, AZ, USA), certified for the use in a transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of complications of portal hypertension. It is made of a triple layer of PTFE and has a self-expanding 10-Fr compatible nitinol skeleton with high radial force and flexibility. The 12 mm diameter that we used in 2004 is no longer available for Viatorr stent-graft. The maximum diameter is now 10 mm due to its efficacy at impeding in-stent TIPS restenosis (10) and the risk of hepatic encephalopathy associated with intrahepatic shunt overflow. However, a wide range of stent-graft is available nowadays. One favourable feature for treating central vein stenosis is that Viatorr has a 2 cm long bare leading end to stabilize the stent without bridging collateral branches. This distal portion was the reason for the femoral deployment of the stent. It enabled treatment of the LBCV stenosis by maintaining venous drainage through the LIJV. A major drawback for the use of stent-grafts in a central vein stenosis is the coverage of ipsilateral internal jugular vein or contralateral brachiocephalic vein. Careful stent-graft selection and placement can potentially avoid an exclusion of patent venous routes providing future accesses in the event of loss of circuit patency (6).
The disappearance of the clinical symptoms of SVCS and CT at 60 months confirmed the efficacy of the stent-graft placement in the presented case. The clinically evaluated primary patency of the stent-graft lasted 94 months until the death of the patient. In conclusion, SVCS with a patent SVC may be causing bilateral jugular or brachiocephalic occlusion due to CVC-related stenosis. The endovascular strategy could be guided by clinical evaluation, angiographic findings and CVC performance. A troublesome substitution of tunnelled catheters is not always necessary. A stent-grafting of the contralateral brachiocephalic vein may be effective without having to act on the CVC in case of a non-infected functioning CVC.
Figures and Tables
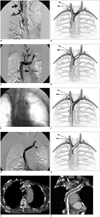
Fig. 1
Angiographic and schematic illustration of procedure.
A. Diagnostic angiography through distal right internal jugular vein injection shows occlusion of right brachiocephalic vein around twin catheter and inverted flow in internal jugular vein (arrow). B. Schematic drawing illustrates initial situation with pericatheter thrombosis involving right internal jugular vein, right subclavian vein and right brachiocephalic vein around well functioning twin catheter. C. Venography from left internal jugular vein reveals critical stenosis of distal left brachiocephalic vein with proximal collateral pathways and patent superior vena cava (arrow). D. Draw illustrates 8 mm predilation of left brachiocephalic lesion performed from left internal jugular vein access subsequently followed by immediate and complete elastic recoil. E. Leading bare end of stent-graft (arrowhead) is open after retrieval of 10-Fr introducer at fluoroscopy snap shot during transfemoral stent-graft releasing. F. Schematic drawing shows free-flow leading end of stent-graft in front of opening to left internal jugular vein. G. Final venography from left internal jugular vein introducer shows direct free flow in right atrium through leading bare end (arrowhead) of Viatorr stent-graft. H. Draw represents final situation with expanded stent-graft permitting flow from left internal jugular vein and left brachiocephalic vein. I. Axial contrast-enhanced multidetector computed tomography performed at 60 months for breast cancer follow-up showing stent-graft (arrow) patency and twin catheter (star). J. Curved multi-planar reconstruction through longitudinal axis of Viatorr stent-graft confirms stent patency (arrowhead) and twin catheter (star) in site.
References
1. Agarwal AK. Central vein stenosis. Am J Kidney Dis. 2013; 61:1001–1015.
2. Riutta JC, Cheville AL, Trerotola SO. SVC syndrome with a patent SVC: treatment of internal jugular venous occlusion after surgical and radiation therapy of esophageal cancer. J Vasc Interv Radiol. 2005; 16:727–731.
3. III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001; 37:1 Suppl 1. S137–S181.
4. Anaya-Ayala JE, Smolock CJ, Colvard BD, Naoum JJ, Bismuth J, Lumsden AB, et al. Efficacy of covered stent placement for central venous occlusive disease in hemodialysis patients. J Vasc Surg. 2011; 54:754–759.
5. Kundu S, Modabber M, You JM, Tam P, Nagai G, Ting R. Use of PTFE stent grafts for hemodialysis-related central venous occlusions: intermediate-term results. Cardiovasc Intervent Radiol. 2011; 34:949–957.
6. Verstandig AG, Berelowitz D, Zaghal I, Goldin I, Olsha O, Shamieh B, et al. Stent grafts for central venous occlusive disease in patients with ipsilateral hemodialysis access. J Vasc Interv Radiol. 2013; 24:1280–1287. quiz 1288.
7. Jones RG, Willis AP, Jones C, McCafferty IJ, Riley PL. Long-term results of stent-graft placement to treat central venous stenosis and occlusion in hemodialysis patients with arteriovenous fistulas. J Vasc Interv Radiol. 2011; 22:1240–1245.
8. Isfort P, Penzkofer T, Goerg F, Mahnken AH. Stenting of the superior vena cava and left brachiocephalic vein with preserving the central venous catheter in situ. Korean J Radiol. 2011; 12:629–633.
9. Stockx L, Raat H, Donck J, Wilms G, Marchal G. Repositioning and leaving in situ the central venous catheter during percutaneous treatment of associated superior vena cava syndrome: a report of eight cases. Cardiovasc Intervent Radiol. 1999; 22:224–226.
10. García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010; 362:2370–2379.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download