Abstract
Objective
To evaluate the safety and clinical efficacy of transcatheter uterine artery embolization (UAE) for post-myomectomy hemorrhage.
Materials and Methods
We identified eight female patients (age ranged from 29 to 51 years and with a median age of 37) in two regional hospitals who suffered from post-myomectomy hemorrhage requiring UAE during the time period from 2004 to 2012. A retrospective review of the patients' clinical data, uterine artery angiographic findings, embolization details, and clinical outcomes was conducted.
Results
The pelvic angiography findings were as follows: hypervascular staining without bleeding focus (n = 5); active contrast extravasation from the uterine artery (n = 2); and pseudoaneurysm in the uterus (n = 1). Gelatin sponge particle was used in bilateral uterine arteries of all eight patients, acting as an empirical or therapeutic embolization agent for the various angiographic findings. N-butyl-2-cyanoacrylate was administered to the target bleeding uterine arteries in the two patients with active contrast extravasation. Technical and clinical success were achieved in all patients (100%) with bleeding cessation and no further related surgical intervention or embolization procedure was required for hemorrhage control. Uterine artery dissection occurred in one patient as a minor complication. Normal menstrual cycles were restored in all patients.
Myomectomy is indicated for patients with symptomatic uterine myomas who wish to preserve their uterus and fertility. The surgical procedure may be performed by laparotomy, laparoscopy or hysteroscopy depending on the location, size, and number of uterine myomas (1, 2, 3).
Hemorrhage associated with myomectomy is not uncommon, and interventions to decrease intra-operative blood loss have been well described (4). Postoperative hemorrhage after myomectomy is a potential complication of this procedure, but the treatment options are not frequently documented in the literature. If refractory to conservative measures, surgery and endovascular procedures are possible options for these patients. Surgery, requiring repair of vessels and uterus, or hysterectomy, has been reported as the definitive treatment for complications related to myomas, such as spontaneous rupture with hemoperitoneum (5) and post-myomectomy spontaneous uterine rupture with severe bleeding (6, 7).
Uterine artery embolization (UAE) has been reported as an effective, minimally invasive treatment option for controlling postpartum hemorrhage (8, 9, 10, 11) and for symptomatic uterine myomas (12, 13, 14, 15, 16). However, the safety and efficacy of UAE for the management of post-operative bleeding after myomectomy have not been well documented as there were only a few published case reports (17, 18, 19, 20, 21, 22). Therefore, we retrospectively review the safety and efficacy of transcatheter UAE using different embolic agents in patients suffering from post-myomectomy hemorrhage.
Retrospective review of eight consecutive female patients from two regional hospitals who suffered from post-myomectomy hemorrhage requiring UAE during the period from 2004 to 2012 was performed. A total of 4860 myomectomies were performed in the two hospitals during the study period: open myomectomies (n = 2400); laparoscopic or hysteroscopic myomectomies (n = 2424); and transcervical myomectomies (n = 36). In eight study patients, the age ranged from 29 to 51 with a median age of 37. Pre-myomectomy sonographic studies of these patients showed that the uterine myomas ranged from 1 to 17 in number and were from 2.5 cm to 11.4 cm in diameter.
Uterine myomectomies were performed to provide symptomatic relief for these patients with uterine myomas. Different approaches were used: open myomectomies (n = 6); hysteroscopic myomectomy (n = 1); and transcervical myomectomy (n = 1). There was no history of prophylactic pre-operative UAE for the uterine myomas. Estimated blood loss during surgery ranged from 100 mL to 1200 mL.
Uterine artery embolization was performed following surgery if there were the following indications: low hemoglobin count (< 13 g/dL) (n = 8); persistent vaginal bleeding (n = 4) or hemodynamic instability refractory to conservative treatment (n = 4). All patients had signs of postoperative hemorrhage with anemia (< 13 g/dL), hypotension (< 100/60 mm Hg) and tachycardia (> 100 beats per minute). The mean hemoglobin level at the time of UAE ranged from 4.5 to 8.5 g/dL, with a mean of 6.8 g/dL. Transfusions of blood products before and after the procedure were performed in all patients, varying from two to nine packs of red blood cells. Vaginal packing was given to one patient and noradrenaline as a vasoconstrictor agent was infused to another patient as decided by the in-charge gynecologists. The time interval between myomectomy and subsequent UAE ranged from 0 to 47 days, with a median of 1.5 days. Of three patients with the time interval of one week or more, one (No. 2) presented with intermittent vaginal bleeding ten days before UAE, another (No. 4) presented with sudden heavy vaginal bleeding one day before UAE, and the remaining one (No. 7) presented with sudden abdominal pain and abdominal distension one day before UAE.
Computed tomography images were obtained in three patients at the same day (No. 4, 7) of UAE or six days (No. 2) before UAE. CT findings were contrast extravasation and peritoneal hematoma in one patient (No. 7) (Fig. 1), multiple pseudoaneurysms within the uterine cavity in another patient (No. 4), and contrast extravasation in the myomectomy site in the remaining patient (No. 2) (Fig. 2).
All UAEs were performed as emergency procedures after informed consents were obtained. Transarterial catheterization using the right transfemoral approach according to the Seldinger technique was used in all cases. Digital subtraction angiography was obtained using 5-Fr Cobra catheters (n = 6), Davis catheter (n = 1), or Roberts uterine catheter (n = 1) in order to outline the pelvic arterial anatomy and to show the bleeding sites. Selective cannulation of bilateral uterine arteries was subsequently performed using 2.2- to 2.4-Fr microcatheters (Renegade; Boston Scientific, Cork, Ireland, or Microferret; Cook, Bloomington, IN, USA) in all patients.
Selective embolizations of the uterine arteries were performed for all patients as empirical and therapeutic measures. Absorbable gelatin sponge particles (Spongostan; Johnson & Johnson, Gauteng, South Africa) were placed under fluoroscopic guidance into bilateral uterine arteries after selective cannulation. N-butyl-2-cyanoacrylate (NBCA, Histoacryl, Braun, Germany) mixed with lipiodol at a ratio of 1:3 was used as a complementary embolic material when there was incomplete hemostasis with the use of gelatin sponge particles. The microcatheter was placed as close as possible to the bleeding site, and the NBCA embolic materials were injected under fluoroscopic guidance into the bleeding arteries. The microcatheter was quickly removed afterward so as to avoid adhesion of the catheter tip to the vessel wall.
Completion pelvic aortographies were performed in all patients after the embolization procedures to ensure hemorrhage cessation and total occlusion of the embolized arteries. All patients were followed up in outpatient clinics 3 to 6 months after the procedure and assessed by gynecologists for post-procedural complications and restoration of menstruation. Post-procedural follow-up imaging at 3 to 6 months were also conducted.
We conducted a retrospective review of the patients' clinical data, pre-operative and post-operative imaging, uterine artery angiographic findings, embolization details, and the clinical outcome.
On the angiographic images, we identified abnormalities of the uterine arteries as well as the presence of pseudoaneurysm or active contrast extravasation. Technically successful embolization was defined as complete occlusion of the target bleeding arteries with no post-procedural contrast extravasation or opacification of the uterine artery pseudoaneurysm. Clinical success of UAE was defined as cessation of vaginal bleeding with no further related surgical intervention or embolization procedure required for hemorrhage control.
The patient characteristics and outcomes are summarized in Table 1.
The pelvic angiography findings were as follows: hypervascular staining without bleeding focus (n = 5) (Fig. 2); active contrast extravasation from the uterine arteries (n = 2, No. 5 and 7, one from the right and one from the left uterine artery) (Fig. 1); and a pseudoaneurysm in the uterus (n = 1, No. 4). When correlated to CT images (n = 3) before UAE, angiographic images were well correlated to CT images obtained at the same day of UAE in two patients (No. 4 and 7) (Fig. 1). On the contrary, contrast extravasation in the myomectomy site on CT scans six days before UAE was not present on angiography in the remaining patient (No. 2) (Fig. 2).
Gelatin sponge particles were administered in bilateral uterine arteries in all eight patients and acted as empirical or therapeutic embolization agents for the various angiographic findings. NBCA was administered to the target bleeding uterine arteries in the two patients (No. 5 and 7) (Fig. 1) with active contrast extravasation refractory to gelatin sponge particles.
Technical success was achieved in all patients (100%) with completion pelvic angiograms showing total occlusion of the target bleeding arteries. Clinical success was achieved in all patients (100%) with successful bleeding control and symptomatic improvement. The anemia in all patients was relieved with significant rise of hemoglobin level after embolization. One patient (No. 7) suffered from uterine rupture before the UAE procedure and required further surgical uterine repair, although hemostasis was achieved after UAE.
Uterine artery dissection occurred in one patient (No. 1) as a minor complication. However, the microcatheter could be negotiated across the dissection and with successful subsequent embolization. There were no procedure-related complications in the remaining patients as assessed by the clinician on follow-up. There was also no pelvic organ ischemia, neuropathy of the sciatic and perineal nerves or post-embolization syndrome in any of the study patients.
Normal menstrual cycles were restored in all patients as documented by the gynecologists during follow-up. Post-embolization follow-up ultrasound (n = 6), CT (n = 1) and MRI (n = 1) imaging showed no further deterioration of pelvic hemorrhage in any of the patients.
Surgery with vessel and uterine repair or hysterectomy has been reported as the definitive treatment for pelvic bleeding related to myomas and post-myomectomy complications including spontaneous uterine rupture with massive hemorrhage (5, 6, 7). Emergent UAE has been determined to be a successful alternative for the management of postpartum hemorrhage (8, 9, 10, 23, 24), but only a few studies have evaluated its use for the treatment of post-myomectomy bleeding. Our study showed 100% technical success with the final angiograms demonstrating total occlusion of the target bleeding arteries. Clinical success was achieved in all patients (100%) as successful bleeding control and symptomatic improvement after UAE.
In previously published studies, UAE has been regarded as a safe procedure with low complication rates varying from 3% to 9% (25, 26, 27, 28). Although UAE can be associated with various complications, including pelvic organ ischemia, neuropathy of the sciatic and perineal nerves, and post-embolization syndrome (29), there was only one case of uterine artery dissection in our study. It was regarded as a minor complication as the uterine artery distal to the dissection could be negotiated with embolization successfully performed.
The patterns of pelvic angiography findings for post-myomectomy hemorrhage are not well-described in the medical literature. In our present study, there were various pelvic angiography findings, the most common being hypervascular staining without bleeding focus, followed by active contrast extravasation from the uterine artery, and pseudoaneurysm in uterus. It is worthwhile to note that uterine pseudoaneurysm can present as a rare but significant cause of delayed bleeding after myomectomy. Therefore, it is important to recognize the various patterns seen in pelvic angiography performed for post-myomectomy hemorrhage. In particular, it has been shown in our study that empirical embolization for patients with only hypervascular staining without bleeding focus will result in a positive clinical outcome and control of post-operative bleeding.
Various embolic agents for UAE have been suggested for controlling bleeding. Absorbable gelatin sponges are the most commonly administered material as they provide temporary occlusion of the selectively cannulated arteries without a major risk of ischemic complications (9, 10). In our study, gelatin sponges were injected into bilateral uterine arteries as an empirical or therapeutic measure and were shown to achieve clinical success in all patients for the control of post-myomectomy hemorrhage. The use of NBCA, a permanent liquid embolic agent, was first reported by Pelage et al. (30) for embolization of pseudoaneurysms with secondary postpartum hemorrhage. Several other studies regarding the use of NBCA for controlling bleeding have subsequently been published (31, 32, 33, 34). Patients with preservation of their fertility following the administration of NBCA have also been reported (31, 33). Our study patient with active contrast extravasation also benefited from the administration of NBCA as hemostasis was achieved without further intervention. This suggests that NBCA may be a useful and potent embolic material, not only for postpartum hemorrhage and uterine artery pseudoaneurysms, but also for controlling post-myomectomy bleeding. Further studies will be helpful to ascertain its role in these cases.
Uterine function and fertility is another important outcome to be determined following UAE. Other studies have shown that UAE is not detrimental to future fertility and pregnancies in patients with postpartum hemorrhage and symptomatic uterine myomas (15, 35, 36). In our study, menstrual cycles were restored in all patients (100%), thus suggesting normal uterine function. However, no fertility data could be obtained in the current study as some patients did not want further conception following their surgery.
Our study has several limitations. As a retrospective study, it has its own inherent limitations. And the sample size may not be large enough to generate a statistically significant conclusion, but such cases are not commonly seen in clinical practice. The impact on fertility after UAE for post-myomectomy bleeding is also not evaluated.
In conclusion, UAE was shown to be a safe, minimally invasive, and effective management option for controlling post-myomectomy hemorrhage without the need for hysterectomy, including patients presented with delayed bleeding. The application of UAE may be extended to the treatment of other gynecological complications other than postpartum hemorrhage and symptomatic uterine myomas.
Figures and Tables
Fig. 1
34-year-old female patient (No. 7) presented with hemodynamic instability.
A. Contrast-enhanced CT scan shows active contrast extravasation within uterine cavity (arrows) and peritoneal space (arrowheads) secondary to uterine rupture after myomectomy. B. Pelvic angiography (anterior-posterior view) confirms presence of active contrast extravasation (arrows). C. Subsequent angiogram (right anterior oblique view) obtained after selective cannulation of left uterine artery shows evidence of active contrast extravasation (arrow). D. Completion pelvic angiography demonstrates cessation of contrast extravasation after successful embolization with N-butyl-2-cyanoacrylate.
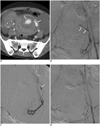
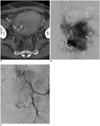
Fig. 2
51-year-old female patient (No. 2) presented with persistent intermittent vaginal bleeding.
A. Contrast-enhanced CT scan six days before uterine artery embolization shows contrast extravasation (arrows) in myomectomy site. B. Selective right uterine arteriogram shows hypervascular staining (arrows) without definite active bleeding focus. Note tortuous right uterine artery (arrowhead). C. Left internal iliac arteriogram shows only tortuous left uterine artery (arrowheads) without bleeding focus. Both uterine arteries were embolized with gelatin sponge particles (not shown).
References
1. Falcone T, Parker WH. Surgical management of leiomyomas for fertility or uterine preservation. Obstet Gynecol. 2013; 121:856–868.
2. Luciano AA. Myomectomy. Clin Obstet Gynecol. 2009; 52:362–371.
3. Lefebvre G, Vilos G, Allaire C, Jeffrey J, Arneja J, Birch C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003; 25:396–418. quiz 419-422.
4. Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2011; (11):CD005355.
5. Lotterman S. Massive hemoperitoneum resulting from spontaneous rupture of uterine leiomyoma. Am J Emerg Med. 2008; 26:974.e1–974.e2.
6. Koyama S, Kobayashi M, Tanaka Y, Isobe M, Miwa H, Shiki Y. Laparoscopic repair of a post-myomectomy spontaneous uterine perforation accompanied by a bizarre tumor resembling polypoid endometriosis. J Minim Invasive Gynecol. 2013; 20:912–916.
7. Pistofidis G, Makrakis E, Balinakos P, Dimitriou E, Bardis N, Anaf V. Report of 7 uterine rupture cases after laparoscopic myomectomy: update of the literature. J Minim Invasive Gynecol. 2012; 19:762–767.
8. Pinto A, Niola R, Brunese L, Pinto F, Losco M, Romano L. Postpartum hemorrhage: what every radiologist needs to know. Curr Probl Diagn Radiol. 2012; 41:102–110.
9. Pelage JP, Le Dref O, Mateo J, Soyer P, Jacob D, Kardache M, et al. Life-threatening primary postpartum hemorrhage: treatment with emergency selective arterial embolization. Radiology. 1998; 208:359–362.
10. Ganguli S, Stecker MS, Pyne D, Baum RA, Fan CM. Uterine artery embolization in the treatment of postpartum uterine hemorrhage. J Vasc Interv Radiol. 2011; 22:169–176.
11. Lee HY, Shin JH, Kim J, Yoon HK, Ko GY, Won HS, et al. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology. 2012; 264:903–909.
12. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003; 79:120–127.
13. Spies JB, Bruno J, Czeyda-Pommersheim F, Magee ST, Ascher SA, Jha RC. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005; 106(5 Pt 1):933–939.
14. Goodwin SC, Spies JB, Worthington-Kirsch R, Peterson E, Pron G, Li S, et al. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008; 111:22–33.
15. Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al. A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess. 2008; 12:1–248. iii.
16. Scheurig-Muenkler C, Lembcke A, Froeling V, Maurer M, Hamm B, Kroencke TJ. Uterine artery embolization for symptomatic fibroids: long-term changes in disease-specific symptoms and quality of life. Hum Reprod. 2011; 26:2036–2042.
17. Ito N, Natimatsu Y, Tsukada J, Sato A, Hasegawa I, Lin BL. Two cases of postmyomectomy pseudoaneurysm treated by transarterial embolization. Cardiovasc Intervent Radiol. 2013; 36:1681–1685.
18. Oishi H, Wada-Hiraike O, Osuga Y, Yano T, Kozuma S, Taketani Y. Spontaneous cessation and recurrence of massive uterine bleeding can occur in uterine artery pseudoaneurysm after laparoscopically assisted myomectomy. J Obstet Gynaecol Res. 2013; 39:598–602.
19. Higón MA, Domingo S, Bauset C, Martínez J, Pellicer A. Hemorrhage after myomectomy resulting from pseudoaneurysm of the uterine artery. Fertil Steril. 2007; 87:417.e5–417.e8.
20. Asai S, Asada H, Furuya M, Ishimoto H, Tanaka M, Yoshimura Y. Pseudoaneurysm of the uterine artery after laparoscopic myomectomy. Fertil Steril. 2009; 91:929.e1–929.e3.
21. Takeda A, Koyama K, Imoto S, Mori M, Sakai K, Nakamura H. Early diagnosis and endovascular management of uterine artery pseudoaneurysm after laparoscopic-assisted myomectomy. Fertil Steril. 2009; 92:1487–1491.
22. Vedantham S, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997; 176:938–948.
23. Rath W, Hackethal A, Bohlmann MK. Second-line treatment of postpartum haemorrhage (PPH). Arch Gynecol Obstet. 2012; 286:549–561.
24. World Health Organization. WHO guidelines for the management of postpartum haemorrhage and retained placenta. Geneva: WHO Press;2009.
25. Kirby JM, Kachura JR, Rajan DK, Sniderman KW, Simons ME, Windrim RC, et al. Arterial embolization for primary postpartum hemorrhage. J Vasc Interv Radiol. 2009; 20:1036–1045.
26. Touboul C, Badiou W, Saada J, Pelage JP, Payen D, Vicaut E, et al. Efficacy of selective arterial embolisation for the treatment of life-threatening post-partum haemorrhage in a large population. PLoS One. 2008; 3:e3819.
27. Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Predictors of failed pelvic arterial embolization for severe postpartum hemorrhage. Obstet Gynecol. 2009; 113:992–999.
28. Diop AN, Chabrot P, Bertrand A, Constantin JM, Cassagnes L, Storme B, et al. Placenta accreta: management with uterine artery embolization in 17 cases. J Vasc Interv Radiol. 2010; 21:644–648.
29. Gonsalves M, Belli A. The role of interventional radiology in obstetric hemorrhage. Cardiovasc Intervent Radiol. 2010; 33:887–895.
30. Pelage JP, Soyer P, Repiquet D, Herbreteau D, Le Dref O, Houdart E, et al. Secondary postpartum hemorrhage: treatment with selective arterial embolization. Radiology. 1999; 212:385–389.
31. Kim GM, Yoon CJ, Seong NJ, Kang SG, Kim YJ. Postpartum haemorrhage from ruptured pseudoaneurysm: efficacy of transcatheter arterial embolisation using N-butyl-2-cyanoacrylate. Eur Radiol. 2013; 23:2344–2349.
32. Matsubara S, Sato T, Nakata M. Vaginal artery embolization with a permanent embolic agent for intractable postpartum hemorrhage. J Obstet Gynaecol Res. 2011; 37:377–378.
33. Igarashi S, Izuchi S, Ishizuka B, Yoshimatu M, Takizawa K. A case of pregnancy and childbirth after uterine artery embolization with a permanent embolic agent. Fertil Steril. 2011; 95:290.e9–290.e11.
34. Kanematsu M, Watanabe H, Kondo H, Goshima S, Kato H, Furui T, et al. Postpartum hemorrhage in coagulopathic patients: preliminary experience with uterine arterial embolization with N-butyl cyanoacrylate. J Vasc Interv Radiol. 2011; 22:1773–1776.
35. Descargues G, Mauger Tinlot F, Douvrin F, Clavier E, Lemoine JP, Marpeau L. Menses, fertility and pregnancy after arterial embolization for the control of postpartum haemorrhage. Hum Reprod. 2004; 19:339–343.
36. Eriksson LG, Mulic-Lutvica A, Jangland L, Nyman R. Massive postpartum hemorrhage treated with transcatheter arterial embolization: technical aspects and long-term effects on fertility and menstrual cycle. Acta Radiol. 2007; 48:635–642.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download