Abstract
This pictorial review provides the principles of extracorporeal membrane oxygenation (ECMO) support and associated CT imaging features with emphasis on the hemodynamic changes and possible imaging pitfalls encountered. It is important that radiologists in ECMO centers apply well-designed imaging protocols and familiarize themselves with post-contrast CT imaging findings in patients on ECMO.
Extracorporeal membrane oxygenation (ECMO) is a modified cardiopulmonary bypass to support life, permitting further treatment and recovery during severe cardiac or pulmonary failure. Several randomized studies have shown ECMO to be a life-saving technique in various clinical settings (1, 2), and its use has expanded dramatically over the past two decades. Furthermore, the applications for ECMO have broadened as techniques and survival rates have improved in pediatric and adult patients.
Computed tomography (CT) is widely applied in the assessment of critical conditions, such as clinical suspicion of complications or an unexplained delay in improvement (3). However, specific hemodynamic changes associated with patients on ECMO determine the contrast-enhanced patterns. It is therefore important that radiologists in ECMO centers are aware of the imaging pitfalls associated with the use of CT in ECMO cases.
We retrospectively evaluate the imaging profiles of patients on ECMO therapy for both thoracic and abdominal CT examinations. We attempt to familiarize radiologists with the hemodynamic changes associated with the various modes of ECMO and the imaging pitfalls of contrast-enhanced CT imaging are emphasized.
Extracorporeal membrane oxygenation is classified primarily into two major types: veno-arterial (VA) type and veno-venous (VV) type. The redistribution of blood flow is significantly different between these two ECMO models. In VA-ECMO, blood is withdrawn from venous circulation, usually via a central vein or the right atrium by gravity siphon. The deoxygenated blood is actively pumped through the oxygenator, and the resulting oxygenated blood is returned to the arterial side of the circulation (typically to the aorta). In VV-ECMO, blood is taken from and returned to the central venous circulation (4). The direction of the aortic bloodstream is reversed in patients on VA-ECMO setting, flowing from the caudal to the cephalad region. In contrast, the aortic bloodstream flows in the normal direction with patients on VV-ECMO. Familiarity of the radiologist with the various types of apparatus positions is essential (Fig. 1).
The hemodynamic changes in patients on VA-ECMO need to be addressed when administering contrast medium. There is retrograde aortic flow driven by the VA-ECMO, which results in inconsistent mixing of the flow from the native heart and the extracorporeal circuit. A variety of situations also cause heterogeneous enhancement after contrast medium injection, depending on the native ejection fraction, the proportion of contrast medium as native preload and inflow to the oxygenator, the inflow rate, the total amount of contrast medium, and the scan time delay for blood redistribution.
During bolus tracking, the region of interest is usually placed over the descending thoracic aorta. This area is a relatively distal part of circulation, located far from the femoral artery where the blood returns from the oxygenator. If scanning is performed prior to complete opacification of the heart chambers, there is a possibility of pseudo-filling defects or contrast-blood layering in the arterial system, including the left heart, aorta, or major arteries (Figs. 2, 3). The contrast-blood levels are suggestive of a weakly pumping heart and loss of pressure gradients between different vascular systems, including arteriovenous and venovenous systems. Hence, the distribution of the injected contrast medium at this time is determined partly by the manually pushed pressure and partly by the hydrostatic pressure of the contrast agent (5). In patients on VA-ECMO with severely impaired left heart function, effective retrograde perfusion requires full bypass with an empty left ventricle and a closed aortic valve (6). Under this condition, the left heart cannot be opacified by injected contrast medium, as shown in Figure 4.
The presence of a poorly opacified aorta or a crescent filling defect within the aorta after contrast enhancement in a patient with a compromised true lumen is considered diagnostic for aortic dissection. Similar findings may be observed in the earlier phase of the CT scan for patients on VA-ECMO. In this situation, the radiologist reporting the CT mistook the pseudo-lesion for an emergency surgical indication (Figs. 3, 4). Therefore, if a filling defect or contrast-blood layering in the arterial system is observed in the post-contrast images, it is necessary to repeat the scanning in an adequately delayed phase (Fig. 2) and correlate with another imaging modality, such as transthoracic or transesophageal echocardiography, to exclude a pseudo-lesion (Fig. 5).
To prevent dilution of contrast medium in the ECMO system, Lidegran et al. (7) proposed administering the contrast agent into the arterial ECMO tubing after the membrane oxygenator, or into the venous line distal to the membrane oxygenator. If possible, it is suggested to reduce pump flow after contrast medium is administrated. Peripheral contrast injection with use of the cannulated limb is not recommended in patients on VA-ECMO due to insufficient circulation and venous return in the distal limbs. The blood flow compromise also occurs in the distal limb of cannulation and may result in arterial ischemia or venous congestion of the distal limb, especially in the setting of prolonged ECMO support. Even in the non-cannulated limb, the venous return is insufficient for contrast injection and it is inadequate for the peripheral administration of contrast medium due to poor cardiac pumping and insufficient perfusion of distal extremities in patients on VA-ECMO (Fig. 6).
Some of the patients on VA-ECMO have mechanical circulatory support devices, such as left ventricular assist devices (LVAD) (Fig. 2) or intra-aortic balloon pumps (IABP) (Figs. 4, 5). The IABP is used to decrease myocardial oxygen consumption by reducing after-load as well as augmenting coronary perfusion during diastole. The LVAD drains the blood directly from the left ventricle and pumps it into the oxygenator or arterial circulation. The circuit is activated by a pump, independent of primary cardiac function, whether preload or afterload. It permits full and stable ventricular unloading (8). In Figure 2, aortic contrast layering was observed in the first scan, but the left ventricle was fully opacified at the same time. The contrast agent in the left heart, probably arising from the native pulmonary circulation and partially driven by LVAD, causes faster contrast opacification than retrograde arterial flow from ECMO does.
Veno-venous ECMO is used in patients with respiratory failure. The cardiocirculatory function in patients on VV-ECMO is normal with the exception of poor pulmonary oxygenation. Dynamic CT images are comparable to those of healthy individuals (Fig. 7).
Figures and Tables
Fig. 1
Diagrammatic representation of (A) VA-ECMO and (B) VV-ECMO.
A. In VA-ECMO, blood is withdrawn from IVC with cannulation via femoral vein (white arrow); after oxygenation, oxygenated blood returns to arterial side of circulation via femoral arterial cannula (black arrow). B. In VV-ECMO, blood is usually taken from SVC (white arrow), driven through oxygenator, and then returned to IVC (black arrow). ECMO = extracorporeal membrane oxygenation, IVC = inferior vena cava, SVC = superior vena cava, VA = veno-arterial, VV = veno-venous
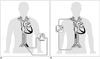
Fig. 2
51-year-old male had acute onset of myocardial infarction during percutaneous coronary intervention under VA-ECMO support.
Contrast-enhanced axial CT images in dynamic phases show contrast layering in descending aorta (white arrow) during arterial phase (A) and full opacification of descending aorta (black arrow) in venous phase (B). Left ventricular assisted device is also shown (arrowhead). VA-ECMO = veno-arterial extracorporeal membrane oxygenation
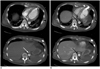
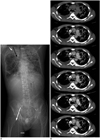
Fig. 3
54-year-old male underwent VA-ECMO due to cardiogenic shock.
(A) In CT topogram, arterial cannula is labeled with black arrow and venous cannula is labeled with white arrow. Contrast medium was administrated via right jugular central venous line (short white arrow). Contrast-enhanced axial CT images in arterial phase (B) and venous phase (C) show pseudo-layering in ascending aorta (white arrows) in both phases and left ventricle appears slightly opacified in second phase. Emergency surgical treatment of suspected thrombi was performed, proving diagnosis of right coronary artery occlusion and myocardial infarction with absence of intimal flap or thrombus formation in ascending aorta. VA-ECMO = veno-arterial extracorporeal membrane oxygenation
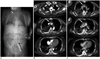
Fig. 4
43-year-old male with acute myocarditis and VA-ECMO support whose ultrasound showed suspected LV and AV thrombus.
(A) Bolus tracking with ROI at ascending aorta is used in order to avoid artifacts from intra-aortic balloon pump (white arrow). Enhancing curve is suggestive of rapid opacification of whole aorta. Contrast-enhanced axial CT images in arterial phase (B) and venous phase (C) show persistent non-enhancement of left ventricular chamber in both phases of scanning. Note full opacification of aortic sinus (white arrows). Further urgent surgical treatment revealed no evidence of intracardiac thrombus. AV = aortic valve, ECMO = extracorporeal membrane oxygenation, LV = left ventricle, ROI = region of interest, VA = veno-arterial
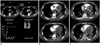
Fig. 5
56-year-old male with underlying congestive heart failure had acute myocardial infarction.
After VA-ECMO was performed, left ventricle assisted device was inserted due to poor cardiac output. A. Post-contrast axial CT scanning during arterial phase shows complete opacification of left carotid artery and left subclavian artery but non-opacification of brachiocephalic artery (black arrow) and fluid layering within ascending aorta (white arrow). Left ventricle is fully opacified. B. During venous phase, filling defects in brachiocephalic artery (black arrow) and aortic root (arrowhead) are outlined by surrounding contrast medium. Fluid layering is persistent as shown at ascending aorta (white arrow). Transesophageal echocardiogram confirmed presence of thrombus at aortic valve and total occlusion of right common carotid artery. VA-ECMO = veno-arterial extracorporeal membrane oxygenation
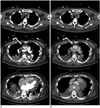
Fig. 6
50-year-old female had sudden cardiac collapse during surgical treatment for brain metastasis from urothelial carcinoma.
VA-ECMO was performed. (A) CT topogram shows arterial cannula at right common femoral artery (black arrow) and venous cannula at right common femoral vein (white arrow). During initial contrast medium injection via peripheral catheter in right arm (short white arrow in right arm), no enhancing flow is detected in bolus tracking with ROI at descending aorta (B). After confirmation of no local extravasation, marked beam-hardening streak artifacts are found due to dense contrast medium stasis in veins of right arm (C, black arrowhead). Upon repeating contrast enhanced scanning, bolus tracking is placed in descending aorta and contrast medium is administrated via central venous line (A, short black arrow in left external femoral vein). Anticipated enhancement curve in descending aorta is obtained (D). Pseudo-layering is also noted at ascending aorta in arterial phase (E, white arrow) as well as delayed opacification of left heart. In venous phase, ascending aorta and left heart are fully opacified by contrast agent (F). ROI = region of interest, VA-ECMO = veno-arterial extracorporeal membrane oxygenation
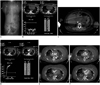
Fig. 7
35-year-old male had pneumonia complicated by acute respiratory distress syndrome and underwent VV-ECMO for oxygenating support.
(A) In CT topogram, inflow cannula is labeled with black arrow and outflow cannula with white arrow. Contrast medium injection is performed via right jugular central venous line (short white arrow). After contrast injection, dynamic serial images are obtained using bolus tracking with ROI at descending aorta and heart chambers and major vessels are enhanced normally (B). ROI = region of interest, VV-ECMO = veno-venous extracorporeal membrane oxygenation
References
1. Bartlett RH, Roloff DW, Custer JR, Younger JG, Hirschl RB. Extracorporeal life support: the University of Michigan experience. JAMA. 2000; 283:904–908.
2. UK Collaborative ECMO Trail Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996; 348:75–82.
3. Miller WT Jr, Tino G, Friedburg JS. Thoracic CT in the intensive care unit: assessment of clinical usefulness. Radiology. 1998; 209:491–498.
4. Zwischenberger JB, Steinhorn RH, Bartlett RH. ECMO: Extracorporeal cardiopulmonary support in critical care. 2nd ed. Ann Arbor, MI: Extracorporeal Life Support Organization;2000. p. 822.
5. Tsai PP, Chen JH, Huang JL, Shen WC. Dependent pooling: a contrast-enhanced sign of cardiac arrest during CT. AJR Am J Roentgenol. 2002; 178:1095–1099.
6. Soeter JR, Smith GT, Anema RJ, Suehiro GT, McNamara JJ. Distribution of oxygenated blood in femoral and brachial artery perfusion during venoarterial bypass in primates. J Thorac Cardiovasc Surg. 1973; 65:825–829.
7. Lidegran MK, Ringertz HG, Frenckner BP, Lindén VB. Chest and abdominal CT during extracorporeal membrane oxygenation: clinical benefits in diagnosis and treatment. Acad Radiol. 2005; 12:276–285.
8. Kay PH, Munsch CM. Techniques in extracorporeal circulation. 4th ed. London: Arnold;2004. p. 354.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download