Abstract
Objective
To compare new full-field digital mammography (FFDM) with and without use of an advanced post-processing algorithm to improve image quality, lesion detection, diagnostic performance, and priority rank.
Materials and Methods
During a 22-month period, we prospectively enrolled 100 cases of specimen FFDM mammography (Brestige®), which was performed alone or in combination with a post-processing algorithm developed by the manufacturer: group A (SMA), specimen mammography without application of "Mammogram enhancement ver. 2.0"; group B (SMB), specimen mammography with application of "Mammogram enhancement ver. 2.0". Two sets of specimen mammographies were randomly reviewed by five experienced radiologists. Image quality, lesion detection, diagnostic performance, and priority rank with regard to image preference were evaluated.
Results
Three aspects of image quality (overall quality, contrast, and noise) of the SMB were significantly superior to those of SMA (p < 0.05). SMB was significantly superior to SMA for visualizing calcifications (p < 0.05). Diagnostic performance, as evaluated by cancer score, was similar between SMA and SMB. SMB was preferred to SMA by four of the five reviewers.
Breast cancer is the most commonly diagnosed malignancy in women (1). Screening mammography is widely recommended for the early detection of breast cancer, and it is the only radiologic method systematically proven to reduce the mortality rate of breast cancer among women aged ≥ 40 years (2, 3). The use of full-field digital mammography (FFDM) for screening purposes has rapidly gained acceptance due to its many advantages over film mammography (4, 5, 6). However, digital mammography is plagued by low sensitivity and high false-positive rates caused by low-contrast characteristics and image noise, while still having the problem of overlapping structures common to traditional projection radiographs (7). Therefore, FFDM manufacturers have focused on improving image quality and have released a post-processing algorithm to increase the contrast resolution of glandular tissues on mammography images (8, 9). This trend was particularly dramatic among patients with malignancies in dense breast tissue.
In Asian countries including Korea, the use of digital mammography has grown in prevalence. Asian women tend to have dense breasts, and digital mammography is known to facilitate detection of cancers obscured by dense breast tissue (10). However, attempts to identify cancer in dense breasts are more likely to yield misses or false positives (7, 11). In this study, we evaluated the performance of the new FFDM alone or in combination with an advanced post-processing algorithm among a population of Korean women. In a previous study, Ko et al. (12) compared new and established FFDM techniques and concluded that diagnostic performance was similar between newer and more established FFDM systems. FFDM developers have recently introduced advanced post-processing algorithms. "Mammogram enhancement ver. 2.0" is a mammographic enhancement algorithm based on wavelet decomposition, which divides an image into several sub-bands containing various features at different scales. No published report has described the image quality obtained when using these new FFDM systems in combination with a post-processing algorithm. Therefore, the aim of our study was to compare use of the new FFDM system alone or in combination with an advanced post-processing algorithm for image quality, lesion detection, diagnostic performance, and reviewer priority ranking.
The institutional review board approved this prospective study, and informed consent was obtained from each patient. We included 100 patients (mean age, 49.3 years; age range, 28.70 years) who had undergone breast surgery at our hospital for excision of a mass or calcification during May 2010 to February 2012. Specimen mammography was performed for all excision specimens during the surgery. Of 100 lesions, 70 were calcifications, 29 were masses with calcifications, and one was a mass without calcification. Fifty-two lesions (52%) were confirmed malignant, and 48 lesions (48%) were benign including 18 high risk lesions (Table 1). Mean calcific lesion size was 2.1 cm (range, 0.2-6.8 cm), and mean mass size was 2.7 cm (range, 1-4.5 cm).
A total of 100 specimens were scanned using a new FFDM system, the Brestige® (Medi-future, Seongnam, Korea). A second type of specimen mammography was processed with an advanced post-processing algorithm ("Mammogram enhancement ver. 2.0"). This allowed us to acquire two sets of mammographic images for each of the 100 specimens: 1) specimen mammography without application of "Mammogram enhancement ver. 2.0" and 2) specimen mammography with application of "Mammogram enhancement ver. 2.0". "Mammogram enhancement ver. 2.0" was a mammographic enhancement algorithm developed by the manufacturer. The flowchart used to represent this algorithm is shown in Figure 1. This algorithm differentiates breast parenchyma and background according to the distribution of brightness in the input image. Image raw data was separated using the multi-scale decomposition technique to increase contrast and sharpness of the image (Fig. 2). Finally, a thickness correction was applied for global dynamic range reduction, resulting in a decreased range of intensity in the breast skin region (Fig. 3). The mean kVp and mAs values for specimen mammography were 28 and 80, respectively. This FFDM system used Tungsten/Rhodium or Tungsten/Argentum as X-ray target and filter. The detector size was 17 × 24 cm and the detector type was direct. The pixel size and image matrix were 85 µm and 2016 × 2816 pixels, respectively.
Five subspecialty-trained breast radiologists with 3, 5, 6, 7, and 11 years of experience, respectively, independently performed a retrospective review of the images. Prior to image analysis, all reviewers were instructed using 12 training cases that were not included in this study, to familiarize each with the standardized protocol for assessing image quality and detecting lesions. There were two interpretation sessions and 100 specimen mammography images were reviewed during each session. To avoid recall bias, the Digital Imaging and Communications in Medicine images from the set of images obtained from a single session were randomized without regard to the device or post-processing algorithm used. A 5-megapixel monitor and a picture-archiving and communication system (Infinitt PACS®, Infinitt Healthcare, Seoul, Korea) were used for the evaluation. All reviewers were blinded to the clinical information and pathologic results of each case; they were also blinded to the ratio of malignant to benign lesions. There was an interval of 2 weeks between review sessions. At each review session, image quality, lesion detection, and diagnostic performance were evaluated for a single set of images.
Image quality was evaluated with respect to overall quality, contrast, and noise. To assess contrast and noise, we adhered to the guidelines proposed by the mammography quality control manual published by the American College of Radiology in 1999 (13). Each of these factors was graded on a 5-step scale (1, not acceptable; 2, poor; 3, moderate; 4, good; 5, excellent). Lesion detection included visibility of calcification, number of calcifications, and mass visibility. Lesion type was assessed using three categories: calcification, mass with calcification, and mass. The visibility of masses and calcifications was classified on a 5-step scale (1, not acceptable; 2, poor; 3, moderate; 4, good; 5, excellent). The number of calcifications was graded on a 5-step scale (1, < 5; 2, 6-10; 3, 11-20; 4, 21-40; 5, > 40).
We applied the cancer scores to determine the diagnostic value of these images. To determine the cancer score, which indicates the probability of malignancy, reviewers scored the images from 0-10. The priority ranking for each image was determined with each mammography protocol. The reviewers looked at two images of the same specimen simultaneously. A low score (score 1) indicated that the image had been ranked above images that received a score of 2.
The statistical analysis was performed using commercially available software (Medcalc, Mariakerke, Belgium). Image quality and lesion detection were compared using the Wilcoxon signed-rank test. The percentages of positive and negative differences between the paired mammography values (specimen mammography A: SMA) and (specimen mammography B: SMB) were also calculated. The difference was positive (or negative) when the value of SMB was greater (or smaller) than that of SMA. The areas under the receiver operating characteristic curves (AUCs) were compared between SMA and SMB to assess the decision to use the cancer score as the reference standard. The reviewers' preference was checked using priority ranking. A p value < 0.05 was considered significant.
Specimen mammography B achieved significantly higher overall quality and contrast than that of SMA by all five reviewers (p < 0.001). SMB was significantly superior to SMA for image noise by all reviewers (p < 0.05). The percentage of positive difference was significantly higher than that of negative difference for all reviewers, for all three factors related to image quality: overall quality, contrast, and noise (Table 2).
Specimen mammography B was significantly superior to SMA for calcification visibility (70 calcifications and 29 masses with calcifications) by all reviewers (p < 0.05) (Fig. 4). However, no significant difference was observed between mammographies with respect to the number of calcifications, for any reviewer. The two sets of mammographies yielded similar ranges of mass visibility values for four reviewers, whereas one reviewer judged that SMB was superior to SMA (Table 3, Fig. 5).
The AUCs of SMA and SMB did not differ significantly with regard to cancer score for any of the reviewers (Table 4).
All five reviewers assigned a lower image preference grade to SMB compared to that of SMA, indicating that all reviewers preferred SMB to SMA (Fig. 6).
Small features such as microcalcifications are prominent in one sub-band, whereas larger features such as masses are dominant in a different sub-band (7). Numerous studies have investigated the utility of wavelet-based enhancement when performing mammography (7, 14, 15, 16). Wavelet-based enhanced digital mammograms are useful for diagnosing calcifications (7, 15, 16). The effects of using this post-processing method to analyze masses have been studied as well (7, 14). Laine et al. (14) suggested that this method could improve image contrast for masses, spicules, and microcalcifications. However, it should be noted that that study was performed using phantom and blended images. However, another report determined no significant improvement when using the post-processing algorithm as compared with unenhanced images (7). In our study, calcification visibility improved with the use of "Mammogram enhancement ver. 2.0", which supports the trends described above. This technique for post-processing facilitates visualization of calcifications. However, no significant difference was observed in terms of mass visibility, as reported previously (7).
The evaluation factors adopted here were derived from protocols used in previous studies (7, 17, 18, 19). Li et al. (17) surveyed 23 studies that clinically evaluated image quality in digital mammography, two of which compared post-processing algorithms. One study attempted to compare lesion visibility (18); the other sought to evaluate overall image quality (7). The studies used a definition of overall image quality that did not include contrast and noise, which were evaluated here. Image quality and lesion detection were graded on a 5-step scale, from not acceptable to excellent, as in previous studies (seven of 23 related studies) (7, 17, 18, 19). Three-step and 7-step scales were used in five and two studies, respectively (17).
We also referred to previous studies to establish a good study design (4, 20). We used the cancer score as described in previous studies. These studies used 7- and 5-point cancer score scales, respectively. We used a 10-point cancer score to increase scoring granularity.
We adhered to the guidelines proposed by Sivaramakrishna et al. (7) and Good et al. (21) for overall statistical analysis and priority rank. In the study by Sivaramakrishna et al. (7), the reviewers judged the priority rank of five enhanced images displayed in random order on three monitors; information regarding the pathology and location of each lesion was available. In this study, we used two monitors at the same time, and mammographic images for two separate specimens were displayed in random order. All reviewers were blinded to the clinical information and pathological results of each case. Good et al. (21) described this process as "multipoint rank ordering". With this method, reviewers rank-order all images for each case from best to worst while the images are displayed side by side. We adopted this method for direct and accurate comparison of the images, with rank was considered to reflect reviewer preference.
Our study had several limitations. First, the object of the study was specimen mammography after surgical excision, which meant that the study population was limited to true patients. Second, most of the lesions included calcifications, which prevented a comprehensive evaluation of the associated masses. In this study, one patient had a mass free of calcification, and 29 patients had masses with calcifications. Finally, there is no designated protocol for evaluating the performance of digital mammography. Based on our literature review, we chose to focus on image quality, lesion detection, diagnostic performance, and priority rank. These factors were chosen because of the availability of data from other studies for comparison.
In conclusion, the reviewers were unanimous in considering specimen mammography with the advanced post-processing algorithm as superior to specimen mammography without the advanced post-processing algorithm in terms of image quality and calcification visibility. In addition, most reviewers preferred the images afforded by specimen mammography with the advanced post-processing algorithm. However, no significant difference was observed between approaches in terms of diagnostic performance.
Figures and Tables
Fig. 4
Specimen mammograms of ductal carcinoma in situ.
Mammography (B) was superior to mammography (A) for visualizing calcifications. However, number of calcifications was determined equally well using both mammography protocols. A. Image scanned by specimen mammography without application of "Mammogram enhancement ver. 2.0". B. Image scanned by specimen mammography with application of "Mammogram enhancement ver. 2.0".
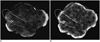
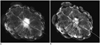
Fig. 5
Specimen mammograms of invasive breast cancer.
Mass was visualized similarly using either mammography protocol. A. Image scanned by specimen mammography without application of "Mammogram enhancement ver. 2.0". B. Image scanned by specimen mammography with application of "Mammogram enhancement ver. 2.0".
References
1. Meisner AL, Fekrazad MH, Royce ME. Breast disease: benign and malignant. Med Clin North Am. 2008; 92:1115–1141.
2. Humphrey LL, Helfand M, Chan BK, Woolf SH. Preventive Services Task Force. Breast cancer screening: a summary of the evidence for the U.S. Ann Intern Med. 2002; 137(5 Part 1):347–360.
3. ACR. Cancer Facts & Figures 2012. American Cancer Society;Published 2012. Accessed May 2, 2013. http://www.cancer.org/.
4. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005; 353:1773–1783.
5. Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008; 246:376–383.
6. Bloomquist AK, Yaffe MJ, Pisano ED, Hendrick RE, Mawdsley GE, Bright S, et al. Quality control for digital mammography in the ACRIN DMIST trial: part I. Med Phys. 2006; 33:719–736.
7. Sivaramakrishna R, Obuchowski NA, Chilcote WA, Cardenosa G, Powell KA. Comparing the performance of mammographic enhancement algorithms: a preference study. AJR Am J Roentgenol. 2000; 175:45–51.
8. Goldstraw EJ, Castellano I, Ashley S, Allen S. The effect of Premium View post-processing software on digital mammographic reporting. Br J Radiol. 2010; 83:122–128.
9. Chen B, Wang W, Huang J, Zhao M, Cui G, Xu J, et al. Comparison of tissue equalization, and premium view post-processing methods in full field digital mammography. Eur J Radiol. 2010; 76:73–80.
10. del Carmen MG, Halpern EF, Kopans DB, Moy B, Moore RH, Goss PE, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007; 188:1147–1150.
11. Jackson VP, Hendrick RE, Feig SA, Kopans DB. Imaging of the radiographically dense breast. Radiology. 1993; 188:297–301.
12. Ko ES, Han BK, Kim SM, Ko EY, Jang M, Lyou CY, et al. Comparison of new and established full-field digital mammography systems in diagnostic performance. Korean J Radiol. 2013; 14:164–170.
13. ACR. Mammography Quality Control Manual. Reston, VA: American College of Radiology;1999.
14. Laine AF, Schuler S, Fan J, Huda W. Mammographic feature enhancement by multiscale analysis. IEEE Trans Med Imaging. 1994; 13:725–740.
15. Strickland RN, Hahn HI. Wavelet transforms for detecting microcalcifications in mammograms. IEEE Trans Med Imaging. 1996; 15:218–229.
16. Kallergi M, Clarke LP, Qian W, Gavrielides M, Venugopal P, Berman CG, et al. Interpretation of calcifications in screen/film, digitized, and wavelet-enhanced monitor-displayed mammograms: a receiver operating characteristic study. Acad Radiol. 1996; 3:285–293.
17. Li Y, Poulos A, McLean D, Rickard M. A review of methods of clinical image quality evaluation in mammography. Eur J Radiol. 2010; 74:e122–e131.
18. Pisano ED, Cole EB, Major S, Zong S, Hemminger BM, Muller KE, et al. Radiologists' preferences for digital mammographic display. The International Digital Mammography Development Group. Radiology. 2000; 216:820–830.
19. Schueller G, Riedl CC, Mallek R, Eibenberger K, Langenberger H, Kaindl E, et al. Image quality, lesion detection, and diagnostic efficacy in digital mammography: full-field digital mammography versus computed radiography-based mammography using digital storage phosphor plates. Eur J Radiol. 2008; 67:487–496.
20. Skaane P, Balleyguier C, Diekmann F, Diekmann S, Piguet JC, Young K, et al. Breast lesion detection and classification: comparison of screen-film mammography and full-field digital mammography with soft-copy reading--observer performance study. Radiology. 2005; 237:37–44.
21. Good WF, Sumkin JH, Dash N, Johns CM, Zuley ML, Rockette HE, et al. Observer sensitivity to small differences: a multipoint rank-order experiment. AJR Am J Roentgenol. 1999; 173:275–278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download