Abstract
Gefitinib is regarded as a relatively safe agent for the treatment of an advanced non-small cell lung cancer (NSCLC). Pulmonary toxicity such as interstitial lung disease associated with gefitinib is uncommon with an estimated all time incidence around 1% worldwide. Moreover, a case of gefitinib associated with pulmonary cystic changes has not been reported yet. In this report we present a case of progressive multiple air cystic changes in both lungs in a patient with NSCLC and intrapulmonary metastases who underwent a gefitinib therapy.
Gefitinib (Iressa, Macclesfield, England) is a selective low-molecular-weight epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) refractory to first and second line therapy (1). Gefitinib is well tolerated and the most adverse drug reactions commonly reported are mild to moderate skin rash, nausea, vomiting and diarrhea. All of them are manageable and non-cumulative (2). Interstitial lung disease (ILD) has been reported in a small minority of patients with gefitinib treatment and is described with an estimated all time incidence of approximately 1% (1). However, the case of gefitinib associated pulmonary cystic changes in NSCLC has not been reported yet. Here, we report the case of a 56-year-old female never-smoker treated with gefitinib for NSCLC with multiple intrapulmonary metastases, who developed progressive diffuse lung parenchymal cystic changes during the treatment.
A 56-year-old female never-smoker initially developed persistent cough, sputum and progressive dyspnea approximately 1 month before the diagnose NSCLC stage IV. The radiograph and computed tomography (CT) of the chest showed a 2.2-cm soft tissue mass in the right upper lobe with multiple small nodules in the bilateral lungs. A case of lung adenocarcinoma with bilateral intrapulmonary metastases was diagnosed. The patient received two courses of systemic chemotherapy with paclitaxel and cisplatin. However, the chemotherapy had to be discontinued due to paclitaxel-induced hypersensitivity reaction. Thereafter the patient refused further chemotherapy. One year later, after receiving two cycles of first-line chemotherapy, the patient visited the emergency room with an abrupt onset of stuttering, quadriparesis and dyspnea. A spontaneous pneumothorax in the left lung and multiple brain metastases were noted. After a chest tube insertion in the left lung and a 30-Gy whole brain irradiation for brain metastases, the treatment with gefitinib was started with a 250-mg daily dosage and continued for the following 15 months because the patient showed an improvement of symptoms and pulmonary function. Although the primary mass in the right upper lobe with multiple metastatic nodules slightly decreased in size after one month gefitinib treatment, the development of new multiple cystic changes was detected on the follow-up high-resolution CT (HRCT) scan. The pulmonary function 9 months after the initiation of gefitinib therapy showed combined severe obstructive and restrictive ventilatory disturbances (predicted forced expiratory volume at timed interval of 1 second [FEV1] 36%, predicted forced vital capacity [FVC] 31%). Due to a poor performance status neither bronchoalveolar lavages nor histological evaluations were available. In contrast to the gradual progression of parenchymal cystic changes in both lungs were on the follow-up chest CT scan a radiographic regression of the main lesion of NSCLC and metastatic nodules noted (Fig. 1A). Simple chest radiographs showed diffuse multiple various sized thin walled cysts and illdefined nodules in bilateral hemi-thorax after the gefitinib therapy. A fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography scan taken 7 months after the use of gefitinib demonstrated a significant increased FDG uptake in the primary lesion only. No uptake was seen in other areas of both lungs, suggesting an interval improvement of metastatic lesions (Fig. 1B). Because the patient's condition was tolerable with improved respiratory symptoms, the therapy with gefitinib was continued for 15 months with the patient's approval, despite of the air cystic changes in both lungs. During the follow-up period, neurologic recovery was noted and the patient underwent pulmonary and physical rehabilitation therapies. A follow-up pulmonary function was performed 13 months after the initiation of gefitinib therapy and more improvement was shown than after 9 months (FEV1, 56%; FVC, 53% vs. FEV1, 36%; FVC, 31%) respectively.
The antineoplastic drug induced pulmonary toxicity caused by drugs, such as cemcitabine, rituximab, fludarabine, paclitaxel, and docetaxel, etc. consists of interstitial lung disease (ILD), i.e., diffuse alveolar and/or interstitial damage, and occurs usually during the first 3 months of treatment (3). The incidence of ILD associated with gefitinib treatment was an all-time around 1% in a recent analysis by the US Food and Drug Administration (2). Among these analyzed cases, 31% had prior radiation therapy and 57% had prior chemotherapy. In Japanese patients the frequency of ILD-type events (1.9%) appears to be higher than in the rest of the world (0.3%). Similar to the present report the most cases were seen in the first month of therapy. Multivariate analysis in previous studies revealed that preexisting pulmonary fibrosis, poor performance status and prior thoracic irradiation were independent risk factors for ILD in patients with gefitinib treatment (4). Although the patient in the presented case had a poor performance status as a factor which induces lung damage, she was a female never-smoker with adenocarcinoma which are known as predictive factors for a good response to gefitinib and showed a tumor regression of lung during the gefitinib therapy also. The patient's clinical background and current status with respect to pulmonary diseases should be considered carefully. However, there is no reason to stop the gefitinib treatment in patients with an inoperable lung cancer (5).
The characteristic HRCT images of gefitinib-induced pulmonary toxicity were similar to those of a drugassociated lung injury (6). Several characteristic HRCT patterns which are seen in other drug-associated ILDs also were observed in the gefitinib-associated condition such as acute interstitial pneumonia-like pattern, cryptogenic organized pneumonia-like pattern, eosinophilic pneumonialike pattern and localized infiltration pattern. The cystic configuration of this case showed similar pattern to the lymphocytic interstitial pneumonia (LIP). An improved lung function after gefitinib use could be partially explained as cystic changes of lung lesions would be transformed into the type of LIP like as the effects of a pulmonary rehabilitation with medications. But the cystic progression of the present case was too fast compared with the usual change of LIP. Also other combined causes of cystic changes have to be considered. To our best knowledge, the presented case is the first report of multiple lung parenchymal cystic changes in a patient treated with gefitinib for NSCLC. Cystic changes by the tumor itself or after a chemotherapy were reported rarely. Tumor necrosis secondary to tumor killing effects in chemosensitive tumors and tumor infiltrations of air-containing spaces with a check-valve mechanism have been postulated for cystic changes (7). Lee et al. (8) reported that squamous cell lung cancer is presenting as an asymptomatic pneumatocele. Barnardt and du Toit (9) reported two cases of cystic metastatic pulmonary lesions, which occurred after chemotherapy and were based on a cisplatinium regimen in an epithelioid trophoblastic tumor and accompanied by pneumothorax as a results of ruptured cavitations. Also the present case showed a spontaneous pneumothorax after chemotherapy before treatment of gefitinib. However, no cysts were found in both lungs at the time of CT evaluation. Also the cystic patterns and the progressing period were unique because the cystic changes widely occurred in both lungs with various sizes. Those changes showed continuous progressive cystic transformations after the use of gefitinib compared with the usual cystic transformation of tumors. Seemingly related to our case partially, Zee et al. (10) reported about a fatal cystic change which occurred in the brain metastasis after gefitinib therapy in a patient with NSCLC. The cytology of the metastatic cystic mass of the brain was negative for malignancy in this study. Cystic changes of tumor sites might have been developed by tumor lysis and necrosis caused by the targeting property of gefitinib towards to cancer cells. This finding is similar to reported case where a cystic formation was developed in the metastatic tumor lesion after the response to gefitinib. Although the cystic changes were persistent the pulmonary function was more improved after 13 months gefitinib therapy then after 9 months. After the treatment with gefitinib the size of the main lesion of right upper lobe with metastatic nodules of both lungs were markedly decreased and cystic changes were developed around the tumor sites. The improvement of lung function could probably be explained as the effect of decrease of tumor burden due to gefitinib and pulmonary medications as well as due to the pulmonary rehabilitation therapy. Therefore, the causes of cystic changes of the presented case may be postulated by an unknown side effect of gefitinib. However, the clear causal relationship of cystic changes with gefitinib remains still unknown, but some potential mechanisms of pulmonary toxicity may be considered. EGFR promotes the regeneration of the alveolar epithelial cells and is up-regulated in an acute lung injury. Consequently, the EGFR inhibitor, gefitinib, may impair normal repair and thereby exacerbate any lung injury, especially in patients with pulmonary comorbidities (11). In addition, it may be considered that the tumor lysis of the main lesion or a lysis on multiple metastatic nodules due to direct cytotoxic effect by gefitinib may have induced the formation of multiple cysts in this case. Within Asia (Korea and Singapore) were found two cases of cystic changes after gefitinib treatment (including the present case) with the involvement of both lungs respective with the involvement of brain (10). Hotta et al. (4) reported 4.5% incidence of ILD associated with gefitinib in Japanese patients. Also high incidences of EGFR somatic mutations of Japanese patients were documented and might be suggested as a contributing factor for the high rate of ILD associated with gefitinib (12).
Therefore it may be assumed that unknown genetic factors such as EGFR somatic mutations might be involved in the development of cystic changes in both cases. We are also aware about a possible close relation between the gefitinib therapy and the onset of illness and suspect gefitinib-induced cystic lung damages. This case showed that progressive multiple cystic changes in both lungs occured during gefitinib therapy besides an ILD.
Figures and Tables
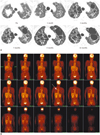 | Fig. 1Lung adenocarcinoma in 56-year-old woman after gefitinib treatment.
A. Lung window of transaxial enhanced CT (5.0-mm collimation) scan obtained at time point of pre-medication and 1 month, 3 months, 7 months, 9 months, and 13 months after initiation of gefitinib treatment. Irregular defined oval shaped 3.2 cm sized mass in right upper lobe with multiple ill-defined nodules of both lungs was gradually decreased in size after use of gefitinib on follow-up chest CT scan in contrast to gradual progression of cystic changes in both lungs. Some cystic lesions are continuously increased in size on follow-up scans during treatment with gefitinib. B. Coronal fluorodeoxyglucose (FDG) positron emission tomography scan obtained after 12 months of treatment demonstrates lesion of markedly increased FDG uptake (standardized uptake value = 5.2, representing primary malignancy) (arrow) without adjacent or distant significant FDG uptake.
|
References
1. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003; 8:303–306.
2. Forsythe B, Faulkner K. Overview of the tolerability of gefitinib (IRESSA) monotherapy: clinical experience in nonsmall-cell lung cancer. Drug Saf. 2004; 27:1081–1092.
3. Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol. 2006; 17:372–379.
4. Hotta K, Kiura K, Tabata M, Harita S, Gemba K, Yonei T, et al. Interstitial lung disease in Japanese patients with non-small cell lung cancer receiving gefitinib: an analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J. 2005; 11:417–424.
5. Teramoto S, Yamamoto H, Ouchi Y. Clinical efficacy and toxicity of gefitinib in patients with lung cancer. Lancet. 2003; 361:1992–1993.
6. Nagaria NC, Cogswell J, Choe JK, Kasimis B. Side effects and good effects from new chemotherapeutic agents. Case 1. Gefitinib-induced interstitial fibrosis. J Clin Oncol. 2005; 23:2423–2424.
7. Topal NB, Oruc E, Gokalp G, Topal U. Atypical pulmonary metastases: radiologic appearances. Indian J Radiol Imaging. 2007; 17:181–185.
8. Lee JC, Kim CH, Koh JS, Baek HJ, Choe DH. Lung cancer presenting as an asymptomatic pneumatocele. Intern Med. 2008; 47:1757–1758.
9. Barnardt P, du Toit J. The radiological appearance of metastatic cystic lesions. SA J Radiol. 2011; 131–133.
10. Zee YK, Chin TM, Wong AS. Fatal cystic change of brain metastasis after response to gefitinib in non-small-cell lung cancer. J Clin Oncol. 2009; 27:e145–e146.
11. Danson S, Blackhall F, Hulse P, Ranson M. Interstitial lung disease in lung cancer: separating disease progression from treatment effects. Drug Saf. 2005; 28:103–113.
12. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download