Abstract
Diffuse pulmonary lymphangiomatosis (DPL) is a rare lymphatic disorder characterized by lymphatic channel proliferation. It is mostly reported in children and young adults. Here, we report a case involving a 52-year-old asymptomatic woman who presented with increased interstitial markings, as seen on a chest radiograph. Diffuse interstitial septal thickening was found on a serial follow-up chest computed tomography scan, and lymphangitic metastasis was the primary radiologic differential diagnosis. However, histologic sections of wedge resected lung revealed diffuse pleural and interlobular septal lymphatic proliferation characteristic of DPL.
Diffuse pulmonary lymphangiomatosis (DPL) is a diffuse lymphatic disease characterized by the proliferation of lymphatic vessels. DPL mostly affects children and young adults with an equal gender prevalence. DPL is a very rare disease, and so far, only five cases have been reported in middle-aged patients in the English-language literature, to our knowledge (1-5).
Computed tomography (CT) findings for DPL include increased interlobular septal thickening, peribronchovascular thickening, patchy ground glass opacities, pleural thickening, pleural effusion, and mediastinal soft tissue infiltration (5). Possible radiologic differential diagnoses include pulmonary edema, pulmonary veno-occlusive disease, Erdheim-Chester disease, lymphangiectasis, lymphangitic carcinomatosis, sarcoidosis, and pulmonary lymphoma.
An increase in the size and the number of anastomosing lymphatic channels in interlobular septa or subpleural areas is seen histopathologically (2). Patients with DPL present with various clinical manifestations and usually have a progressive clinical course (2).
Here, we describe a middle-aged woman with DPL in whom clinical suspicion of lymphangitic metastasis was raised preoperatively.
A 52-year-old woman with abnormal chest radiographs was referred to our hospital. She was asymptomatic and denied having any cough, wheezing, or hemoptysis. Her past medical history was unremarkable. The findings of a physical examination were normal. Laboratory examination revealed a hemoglobin level of 14.1 g/dL, a white blood cell count of 8160/µL (61.7% neutrophils, 31.1% lymphocytes, 2.6% eosinophils, 4.4% monocytes, and 0.2% basophils), and a platelet count of 209000/µL. Urine analysis findings, blood chemistry findings, and erythrocyte sedimentation rates were normal.
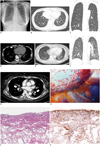
Posteroanterior chest radiograph showed increased interstitial markings in both lungs (Fig. 1A). Diffuse, smooth, and nodular interlobular septal thickening, and minimal amounts of bilateral pleural effusion were demonstrated on CT scan (Fig. 1B, C). Low-density infiltration of mediastinal fat and lymph node enlargement were noted in the right anterior diaphragmatic area of a mediastinal window (Fig. 1D). Although the patient was asymptomatic, these imaging findings persisted on the follow-up CT scan taken one month later. Our primary radiologic impression was that this was a case of lymphangitic carcinomatosis. Pulmonary edema, sarcoidosis, and lymphoma were included in the differential diagnosis.
A pulmonary function test showed mild restrictive patterns. The patient underwent bronchoscopy, and no endobronchial lesion was found. Bronchoalveolar lavage results were negative for malignant cells. Thick varicose septal veins and intraalveolar macrophages were identified on transbronchial lung biopsy.
After seven months, a follow-up chest CT scan revealed increased interstitial septal thickening (Fig. 1E, F), new peribronchovascular cuffing, and small amounts of bilateral pleural effusion (Fig. 1G). Finally, the patient underwent video-assisted thoracoscopic surgery wedge resection of the right, middle, and lower lobes. In the surgical field, abnormal hypervascularity was noted on the lung surface (Fig. 1H).
Microscopic examination showed proliferation of thinwalled, anastomosing vascular spaces lined by a single layer of endothelial cells lacking cytological atypia (Fig. 1I). Theses lesions were located along the lymphatic distribution and were highlighted by D2-40 (1:100, Dako, Glostrup, Denmark) immunohistochemical stain (Fig. 1J), characteristic of DPL. Presently, the patient is alive without any symptoms and being observed without specific treatment such as low fat medium chain fat.
Diffuse pulmonary lymphangiomatosis is a rare condition in which diffuse proliferation of anastomosing lymphatic channels is observed. It manifests equally in both sexes, mostly in children and young adults (3). Symptoms include dyspnea, nonproductive cough, bronchial casts, milky sputum, fever, and recurrent pneumonia. However, patients can present with the disease later in adulthood and often have symptoms dating back to their childhood (5).
To the best of our knowledge, only five cases of DPL in middle-aged patients have been reported in the English literature (Table 1) (1-5), with the current report being the sixth. Ours is a unique one in the following aspects; although the disease progression revealed on serial follow-up CT required differentiation from lymphangitic metastasis, the patient remained asymptomatic and did not need any treatment; also, the extent of the disease markedly increased within one year, and a neoplastic but possibly benign component was presumed.
Making a preoperative diagnosis of DPL in adults is difficult. The most common radiologic finding is increased interstitial markings on chest radiography. CT demonstrates smooth or nodular interlobular, septal, and peribronchovascular thickenings. Patchy ground glass opacities are also seen. Pleural thickening, pleural effusion, and diffuse mediastinal soft tissue infiltration can also occur (5).
Pulmonary edema, pulmonary veno-occlusive disease, Erdheim-Chester disease, and lymphangiectasis can be considered possible diagnoses when smooth interlobular septal thickening is found. In this patient, pulmonary edema was less likely since there was no evidence of congestive heart failure or pleural effusion. Prominent central pulmonary arteries were also not identified, making pulmonary veno-occlusive disease less likely (6). The possibility of Erdheim-Chester disease, characterized by proliferation of lipid-containing foamy histiocytes in the skeleton and other organs, was ruled out by the absence of sclerotic changes in the diaphyses and metaphyses of long bones in the current case (7).
The CT appearance of DPL is virtually identical to that of pulmonary lymphangiectasia (8). Pulmonary lymphangiectasia is a rare condition characterized by the diffuse dilatation of pulmonary lymphatics and classified as congenital or secondary (9). In this case, there was no evidence of pulmonary hypertension or venous obstruction, factors that can cause secondary lymphangiectasia. Congenital lymphangiectasia typically presents shortly after birth and is associated with high neonatal morbidity and mortality. Lymphangiomatosis typically presents in older children and is rarely seen in adults (10). Histopathologically, lymphangiomatosis is characterized by an increased number of variable-sized lymphatic spaces. This should be distinguished from the findings of lymphangiectasia, in which nonproliferative lymphatic channels are dilated (4).
Other diseases with lymphatic distributions, such as lymphangitic carcinomatosis, sarcoidosis, and pulmonary lymphoma, can also be considered. In these diseases, interlobular septal thickening tends to be nodular.
There is no established treatment for DPL. Surgical resections can be indicated for localized mediastinal or lung lesions. Other treatments include low-fat medium-chain triglyceride diets, interferon-alpha, radiation, corticosteroids, chemotherapy, somatostatin, and propranolol (2).
We revealed an unusual presentation of DPL in a middle-aged asymptomatic woman. In follow-up imaging studies, the extent of the disease had increased, and a surgical biopsy was performed to rule out lymphangitic carcinomatosis. An awareness of the possibility of DPL even in adults is necessary.
Figures and Tables
Fig. 1
52-year-old woman with diffuse pulmonary lymphangiomatosis.
Posteroanterior chest radiograph revealing increased interstitial markings in both lungs (A). Initial CT scan demonstrating diffuse interstitial thickening in both lungs (B, C) and lymph node enlargement in right anterior diaphragmatic area (D, arrow). Follow-up CT taken eight months after initial CT scan showing increased interlobular septal thickening (E, F) with more prominent bronchovascular bundle thickening (arrows) and increased bilateral pleural effusion (G). Intra-operative photograph showing hypervascularity of lung surface (H).
I. Hematoxylin and eosin-stained section of lesion showing proliferation of thin-walled, anastomosing lymphatic vessels lined by single layer of endothelial cells lacking cytological atypia (arrows, × 200). J. Immunohistochemical staining with D2-40 revealing proliferative lymphatic channels (arrows, × 200).
References
1. Swensen SJ, Hartman TE, Mayo JR, Colby TV, Tazelaar HD, Müller NL. Diffuse pulmonary lymphangiomatosis: CT findings. J Comput Assist Tomogr. 1995; 19:348–352.
2. Yoo SH, Song JS, Lee JJ, Lee M, Hwang HS, Jang SJ. Diffuse pulmonary lymphangiomatosis: Pulmonary lymphatic disorder in an adult. Basic Appl Pathol. 2012; 5:63–66.
3. El Hajj L, Mazières J, Rouquette I, Mittaine M, Bolduc JP, Didier A, et al. Diagnostic value of bronchoscopy, CT and transbronchial biopsies in diffuse pulmonary lymphangiomatosis: case report and review of the literature. Clin Radiol. 2005; 60:921–925.
4. Boland JM, Tazelaar HD, Colby TV, Leslie KO, Hartman TE, Yi ES. Diffuse pulmonary lymphatic disease presenting as interstitial lung disease in adulthood: report of 3 cases. Am J Surg Pathol. 2012; 36:1548–1554.
5. Du MH, Ye RJ, Sun KK, Li JF, Shen DH, Wang J, et al. Diffuse pulmonary lymphangiomatosis: a case report with literature review. Chin Med J (Engl). 2011; 124:797–800.
6. Resten A, Maitre S, Humbert M, Rabiller A, Sitbon O, Capron F, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol. 2004; 183:65–70.
7. Wittenberg KH, Swensen SJ, Myers JL. Pulmonary involvement with Erdheim-Chester disease: radiographic and CT findings. AJR Am J Roentgenol. 2000; 174:1327–1331.
8. Copley SJ, Coren M, Nicholson AG, Rubens MB, Bush A, Hansell DM. Diagnostic accuracy of thin-section CT and chest radiography of pediatric interstitial lung disease. AJR Am J Roentgenol. 2000; 174:549–554.
9. Nobre LF, Müller NL, de Souza Júnior AS, Marchiori E, Souza IV. Congenital pulmonary lymphangiectasia: CT and pathologic findings. J Thorac Imaging. 2004; 19:56–59.
10. Tazelaar HD, Kerr D, Yousem SA, Saldana MJ, Langston C, Colby TV. Diffuse pulmonary lymphangiomatosis. Hum Pathol. 1993; 24:1313–1322.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download