Abstract
Objective
To assess the feasibility of computed tomography (CT) lymphography using ethiodized oil for sentinel node mapping in experimentally induced VX2 carcinoma in the rabbit thigh.
Materials and Methods
This experiment received approval from the institutional animal use and care administrative advisory committee. Twenty-three rabbits with VX2 carcinoma in the thigh underwent CT before and after (1 hour, 2 hour) peritumoral injection of 2 mL ethiodized oil. After the CT examination, sentinel nodes were identified by peritumoral injection of methylene blue and subsequently removed. The retrieved sentinel and non-sentinel lymph nodes were investigated with radiographic and pathologic examinations. Based on the comparison of CT findings with those of radiographic and pathologic examinations, the diagnostic performance of CT for sentinel node identification was assessed.
Results
All 23 rabbits showed 53 ethiodized oil retention nodes on post-injection CT and specimen radiography, and 52 methylene blue-stained nodes at the right femoroiliac area. Of the 52 blue-stained sentinel nodes, 50 nodes demonstrated ethiodized oil retention. Thus, the sentinel node detection rate of CT was 96% (50 of 52). On pathologic examination, 28 sentinel nodes in 17 rabbits (nodes/rabbit, mean ± standard deviation, 1.7 ± 0.6) harbored metastasis. Twenty seven of the 28 metastatic sentinel nodes were found to have ethiodized oil retention.
As the sentinel lymph node is most likely to be the initial site of regional node metastases, the possibility of metastases in other lymph nodes are very unlikely when the sentinel node is negative for metastasis (1). Sentinel node navigation surgery with radiocolloid or vital dye injection has been widely used as an alternate, less-invasive surgical procedure to traditional wide lymph node dissection for early stage breast cancer, malignant melanoma, and early gastric cancer (1-7). Recently, some investigators reported that computed tomography (CT) lymphography using a water soluble contrast agent could visualize lymphatic drainage pathways from the primary tumor to sentinel nodes and provide detailed anatomy of the sentinel node based on CT images (8-13). However, since the water soluble contrast agent, iopamidol, rapidly enhances lymph nodes and washes out of lymph nodes, the optimal time window for sentinel node detection is very short and the resected sentinel nodes cannot be directly matched with the detected nodes on CT (8-13).
Ethiodized oil (Lipiodol Ultra-Fluid; Andre Guerbet, Aulnay-sous-Bois, France) has been used for conventional lymphography owing to their characteristic of preferential passage along lymphatics and retention in lymph nodes. Kim et al. (14) demonstrated the feasibility of CT lymphography using ethiodized oil for sentinel node mapping in patients with early gastric cancer. After peritumoral submucosal injection of ethiodized oil, CT could successfully visualize sentinel nodes with ethiodized oil retention. However, the feasibility of CT lymphography with peritumoral injection of ethiodized oil for soft tissue tumor including breast cancer and soft tissue sarcoma has not been demonstrated. VX2 tumor has been used to study the behaviors of various soft tissue tumors, including bone tumor, soft tissue sarcoma (15) and breast cancer (16) in rabbit models. Thus, the purpose of this experimental study was to assess the feasibility of interstitial CT lymphography using ethiodized oil for sentinel node mapping of experimentally induced VX2 carcinoma in the rabbit thigh.
Approval for this study was obtained from the animal use and care administrative advisory committee of our institution. All experiments were conducted in accordance with the general guidelines issued by the National Institutes of Health for the care of laboratory animals.
The pilot study for CT lymphography and identification of sentinel node was performed in two adult New Zealand white rabbits weighing between 3.0 to 3.5 kg. The animals were sedated with an intravenous injection of a mixed solution of ketamine hydrochloride (50 mg per kg of body weight, Ketalar; Yuhan Yanghaeng, Seoul, Korea) and 2% xylazine hydrochloride (0.1 mL per kg of body weight, Rompun; Bayer, Seoul, Korea). Tumor implantation was performed with an aseptic technique; VX2 carcinoma was inoculated in the muscle at the right upper thigh by injecting 0.5 mL of tumor suspension with an 18-gauge needle. The experimental VX2 carcinoma was prepared in a manner reported previously (17). Tumor growth was monitored by ultrasonography (HDI-3000; Advanced Technology Laboratories, Bothel, WA, USA) with a 10-5-MHz linear transducer. VX2 carcinoma nodules were allowed to grow to larger than 2.5 cm in diameter, which typically required 14 to 20 days, before CT examination.
For CT imaging, the rabbits were fixed in a supine position on a board made of rigid paper. The CT examinations were performed by using a multidetector row CT scanner (Lightspeed Ultra; GE Medical Systems, Milwaukee, WI, USA). Before peritumoral injection of ethiodized oil (Lipiodol Ultra-Fluid; Andre Guerbet, Aulnay-sous-Bois, France), noncontrast CT images (pre-injection CT) were obtained with a scan coverage from the diaphragm to the knee joint, in order to distinguish the nodal uptake of ethiodized oil from pre-existing nodal or vascular calcification. Helical scan data were acquired using 8 × 1.25 mm collimation, a rotation speed of 0.5 seconds, a table speed of 13.5 mm/rotation, 120 kVp, and 200 effective mAs. A reconstruction section thickness of 2.5 mm and a reconstruction interval of 1.25 mm were used. After obtaining the pre-injection CT, peritumoral ethiodized oil injection was performed under ultrasonographic guidance. In the first rabbit, a total of 1 mL of ethiodized oil was injected into four peritumoral areas (upper; lower; right; left) with a 24-gauge needle attached to 5-mL volume syringe. In the second rabbit, a total of 2 mL of ethiodized oil was injected into the four peritumoral areas. After ethiodized oil injection, the injection sites were gently massaged for one to two minutes in order to facilitate the migration of the ethiodized oil to the draining nodes (8). Post-injection noncontrast CT images with the same protocol for pre-injection CT were obtained at 30 minutes and 1 hour after ethiodized oil injection. The nodular or curvilinear foci of ethiodized oil retention were only shown in the right femoroiliac nodes of the second rabbit. After obtaining post-injection CT, sentinel node identification was attempted. After making a long incision from the epigastric area to the right thigh, methylene blue was injected into the same four peritumoral areas as ethiodized oil injection. A total of 1 mL and 2 mL of methylene blue was injected in the first and the second rabbit, respectively. After 10 minutes, the right femoroiliac nodes began to be blue-stained only in the second rabbit. Therefore, we decided to inject a total of 2 mL of ethiodized oil and methylene blue, respectively, for the main study.
A total of 23 adult New Zealand white rabbits weighing between 3.0 to 3.5 kg were used. The animals were prepared in the same manner as the pilot study for sedation, VX2 carcinoma inoculation and the acquisition of CT images. Under ultrasonographic guidance, a total of 2 mL of ethiodized oil was injected into four peritumoral areas (upper; lower; right; left) with a 24-gauge needle attached to a 5-mL volume syringe. The volume of ethiodized oil was equally divided into these four injection sites. After injection of the ethiodized oil, the injection sites were gently massaged for one to two minutes in order to facilitate the migration of the ethiodized oil to the draining nodes (8). Post-injection noncontrast CT images with the same protocol for pre-injection CT were obtained at 1 (post-1h CT) and 2 hours (post-2h CT) after ethiodized oil injection.
After post-2h CT examination, a total of 2 mL of 1% methylene blue was injected into the same four peritumoral areas as ethiodized oil injection, followed by one to two minutes of gentle massage at the injection site. Sentinel node biopsy was begun within 10 to 15 minutes after methylene blue injection (2, 18, 19). After a long incision from the epigastric area to the right thigh was made, sentinel nodes were identified by tracing the blue-stained lymphatics that were draining to the blue-stained nodes. Following the identification of blue-stained nodes, all animals were euthanized by using a rapid intravenous injection of a lethal amount of sodium thiopental (Pentothal; Choong Wae Pharmacy, Seoul, Korea), and the right femoroiliac region including the blue-stained nodes was resected. A soft X-ray radiographic unit (Faxitron 43805N; Hewlett-Packard, Sunnyvale, CA, USA) and X-Omat V film (Kodak, Rochester, NY, USA) were used to perform specimen radiography for the removed lymphatic basin. Radiographs of the specimens were obtained with technical parameters of 25 kVp, 3 mA, and 3-4-minute exposure. After radiographic examination, the blue-stained lymph nodes and other non-stained nodes were extirpated from the lymphatic basin; then, other lymph nodes were removed to the paraaortic area. The number and the location of the identified nodes were recorded. All removed lymph nodes were submitted as frozen section for lipid (Oil-Red-O) staining and hematoxylin-eosin staining at 2-mm interval section.
Computed tomography image datasets were transferred into a workstation (Rapidia; INFINITT, Seoul, Korea) and reviewed by two radiologists in consensus. The lymph node containing nodular or curvilinear high attenuation foci was regarded as the ethiodized oil retention node on post-injection CT. The number and location of ethiodized oil retention nodes were recorded. According to the location, the ethiodized oil retention nodes were labeled in alphabetical order along the craniocaudal direction. The quantitative analysis included the measurement of the attenuation value in Hounsfield units (HU) of each lymph node containing ethiodized oil. The attenuation value was measured on the transverse images of pre-injection and two sets of post-injection CT at a window width and level setting of 400 HU and 20 HU, respectively. For the attenuation measurement, circular regions-of-interest (ROI) were manually drawn by consensus of the two reviewers in each ethiodized oil retention node identified on either post-1h or post-2h CT while the area of ROI was adapted to encompass as much of the ethiodized oil retention node as possible. On the same animal, the size and location of the ROI were kept constant for measurement at different time points. On the radiography of the removed lymphatic basin, a nodular structure with high attenuation foci was regarded as the ethiodized oil retention node. The number and the location of lymph nodes detected on radiography were recorded in the same manner as with the CT images. The number and the location of ethiodized oil retention nodes from CT images were compared to those from sentinel node mapping after methylene blue injection and from the radiographic images of the specimen.
The histologic sections for removed lymph nodes were reviewed by a pathologist. In the lipid (Oil-Red-O)-stained section, the presence and location of ethiodized oil were determined. For the hematoxylin-eosin-stained sections, the presence of metastatic tumor was identified.
The sentinel node detection rate of CT lymphography was defined as the number of lymph nodes detected by CT lymphography divided by the number of methylene blue-stained nodes. A failure of CT lymphography in identifying a sentinel node was defined as the absence of tumor cells in the sentinel node (negative sentinel node) while other non-sentinel nodes harbored tumor cells in certain rabbits (14). Based on the histologic examination of the removed lymph nodes, the sentinel node detection rate and the number of cases in which CT lymphography failed to identify a sentinel node were assessed. The significance of the difference in attenuation values among pre-injection, post-1h and 2h CT images was assessed by using repeated measures analysis of variance with a Greenhouse-Geisser correction and post hoc tests using the Bonferroni correction. IBM SPSS Statistics version 20 (IBM, Armonk, NY, USA) was used to analyze the data. A p-value < 0.05 was considered to indicate a statistically significant difference.
There were no high attenuation foci such as calcification within the lymph nodes on pre-injection CT images. The nodular or curvilinear foci of ethiodized oil retention were shown in 46 right femoroiliac nodes of 20 rabbits (range, 1-3 nodes/rabbit; mean ± standard deviation [SD], 2.3 ± 0.8) on post-1h CT images and 53 nodes of 23 rabbits (range, 1-4 nodes/rabbit; mean ± SD, 2.3 ± 0.8) on post-2h CT images. In all 23 rabbits, a total of 52 blue-stained sentinel nodes (range, 1-3 nodes/rabbit; mean ± SD, 2.3 ± 0.8) were successfully identified after methylene blue injection. All blue-stained nodes were located at the right femoroiliac region. On the specimen radiography of the removed specimen, a total of 66 lymph nodes (range, 1-4 nodes/rabbit; mean ± SD, 2.9 ± 0.6) were detected at the right femoroiliac region. Among them, 53 lymph nodes (range, 1-4 nodes/rabbit; mean ± SD, 2.3 ± 0.8) demonstrated nodular or curvilinear ethiodized oil retention. The ethiodized oil retention nodes on the radiography of blue-stained basin were well matched with those on post-injection CT images. Among 52 blue-stained lymph nodes, 50 lymph nodes showed ethiodized oil retention on radiography. CT lymphography demonstrated three additional ethiodized oil retention nodes without methylene blue uptake. Therefore, the sentinel node detection rate for blue-stained sentinel nodes by CT lymphography was 96% (50 of 52 blue stained nodes) (Fig. 1). The mean attenuation values of the ethiodized oil retention nodes in HU were 42.2 ± 4.9 HU (95% confidence interval [CI], 40.9 to 43.5; range, 33.8-57.3) on pre-injection CT images, 70.3 ± 32.8 HU (95% CI, 61.7 to 78.8; range, 41.6-200.4) on post-1h CT images, and 144.7 ± 76.1 HU (95% CI, 124.9 to 164.5; range, 59-407.5) on post-2h CT images. The attenuation values on CT images differed according to the time points with statistical significance (F [1.248, 72.384] = 90.979, p < 0.0005). Post hoc tests using the Bonferroni correction revealed that the differences of CT attenuation values between all three pairs of time points were statistically significant (p < 0.0005, respectively) (Fig. 2).
The flow diagram of the histologic findings of retrieved lymph nodes is depicted in Figure 3. A total of 103 lymph nodes (range, 3-6 nodes/rabbits; mean ± SD, 4.5 ± 0.7) were retrieved from 23 rabbits. Among them, 68 lymph nodes (range, 2-4 nodes/rabbit; mean ± SD, 3.0 ± 0.5) were obtained from blue-stained lymphatic basin in the right femoroiliac area and other 35 lymph nodes were from other areas of 23 rabbits. Metastasis was found in 32 lymph nodes of 17 rabbits (range, 1-4 nodes/rabbit; mean ± SD, 1.9 ± 0.9). Of these 32 metastatic nodes, 28 nodes (range, 1-3 nodes/rabbit; mean ± SD, 1.7 ± 0.6) were found in the retrieved sentinel basin of 17 rabbits while the remaining four metastatic nodes were detected in non-blue stained basin of four rabbits. Of the 28 sentinel nodes that harbored metastasis, 27 lymph nodes of 17 rabbits (range, 1-3 nodes/rabbit; mean ± SD, 1.6 ± 0.6) had ethiodized oil retention. One metastatic sentinel node did not have ethiodized oil retention; however, another metastatic node in the same rabbit had ethiodized oil retention. Therefore, the per-rabbit detection rate of ethiodized oil retention in the metastatic sentinel nodes was 100% (17 of 17 rabbits).
All 53 ethiodized oil retention nodes detected on both post-injection CT and specimen radiography showed intranodal lipid deposit mainly in the subcapsular sinusoids and partly in the medullary sinusoids on Oil-red-O stain. There was no evidence of architectural distortion of the lymph node by the retained ethiodized oil.
In our experimental model for soft tissue tumor, we could demonstrate that ethiodized oil injected at peritumoral areas accumulated in most of the sentinel lymph nodes (50 of 52 nodes), including most of those harboring metastasis (27 of 28 nodes; 17 of 17 rabbits), in amounts demonstrable on CT. This result was in accordance with the previous report that demonstrated the feasibility of CT lymphography using ethiodized oil for patients with early gastric cancer (14). Although the lymphatic drainage of the gastrointestinal tract is more complicated than superficial soft tissue tumors and result in more frequent skip metastasis, one unfavorable feature of sentinel lymph node detection, this previous study showed promising results. After submucosal injection of ethiodized oil in patients with early gastric cancer, CT could demonstrate the ethiodized oil retention nodes in perigastric areas with detailed anatomic information, and sentinel basin extirpation was successfully performed according to the anatomic information of the sentinel basin provided by CT lymphography. There was no patient with metastasis in other non-sentinel lymph nodes without metastatic foci in the sentinel basin (14). Given that the goals of the sentinel node navigation surgery is avoiding unnecessary wide nodal dissection with preservation of oncologic safety, our result that demonstrated 100% per-rabbit detection rate of ethiodized oil retention in metastatic sentinel nodes suggests that CT lymphography using ethiodized oil may be a feasible new technique for preoperative sentinel node mapping for soft tissue tumor.
Certain histologic subtypes of soft tissue tumors such as epithelioid sarcoma, rhabdomyosarcoma, and clear cell sarcoma are known to have a higher frequency of lymph node metastases, ranging from 17% to 50% (20, 21), which is substantially higher than the overall prevalence of lymph node metastasis in soft tissue sarcoma, which is reportedly 1.75% to 5.9% (20-22). Although the survival benefit of regional lymphadenectomy for soft tissue sarcoma is still undetermined, a recent retrospective study comparing patients with nodal metastases treated with and without lymphadenectomy reported improved survival at 1.5 years in those managed with lymphadenectomy, but subsequently no difference at 5 years (23). Sentinel lymph node biopsy has been demonstrated to be helpful for detecting clinically occult regional lymph node metastasis for patients with clear cell carcinoma from a prospective trial including 62 consecutive patients with soft tissue sarcoma (24). Therefore, our method of sentinel node mapping with CT lymphography may be valuable in the preoperative work up of soft tissue sarcoma patients, especially for those with histologic subtypes at high risk of lymph node metastasis.
It has been well established that sentinel node navigation surgery using radiocolloid scintigraphy with intraoperative gamma probe counting and vital blue dye methods is clinically useful in patients with melanoma and breast cancer with a high success rate of more than 95% and less than 3.4% rate of failure in identifying a sentinel node (1-6, 25). However, these conventional methods for sentinel node detection have some fundamental drawbacks. The limited spatial resolution of the lymphoscintigraphy impedes with providing accurate anatomic information in the preoperative evaluation. In addition, intraoperative gamma-probe counting technique has inherent subjectivity in the detection method itself, which requires the operator some experience for confident sentinel node detection. Moreover, the high radioactivity at the primary injection site can hinder the detection of radioactive hot nodes by gamma probe (3-5, 26-29). Furthermore, these conventional methods are only feasible in the intraoperative period. Even though our study used an experimental model, CT lymphography using peritumoral injection of ethiodized oil could show 100% technical success rate without any cases in which CT lymphography failed to identify a sentinel node. Metastasis could be found in 27 of 53 ethiodized oil retention nodes and five of 50 non-ethiodized oil retention nodes, while there were no metastatic foci in other non-ethiodized oil retention nodes when ethiodized oil retention nodes were negative for metastasis on a single rabbit basis. Also, the detection rate of blue-stained nodes by CT lymphography was 96%, which is comparable to the previous results (1-6, 25). Moreover, CT lymphography using ethiodized oil has an advantage of longer time window for sentinel node detection, compared to water soluble contrast agent such as iopamidol, which rapidly enhances and washes out of lymph nodes, giving a short time window for detection and making it impossible to directly match the resected nodes with the detected nodes on CT (8-13).
After peritumoral injection of ethiodized oil, post-2h CT could demonstrate seven additional ethiodized oil retention nodes than post-1h CT in three rabbits, which could only be shown in post-2h CT. Also, the attenuation values of ethiodized oil retention nodes on post-2h CT were significantly higher than that on post-1h CT. In our study, ethiodized oil injected in peritumoral areas migrated into the lymph nodes via lymphatics. The migration of interstitially administered lipid substances including ethiodized oil into initial lymphatics can be accomplished either by an extracellular pathway through the openings between endothelial junctions or by an intracellular pathway through phagocytes (30, 31). Although the ethiodized oil can be trapped by and migrated with phagocytes (32), the lymphatic intracellular pathway via phagocytosis usually requires more time than the extracellular pathway because the intracellular transport needs cascades such as localization of phagocytes, phagocytosis and migration into the initial lymphatics (31). Given that post-injection CT examinations were performed one and two hours after peritumoral injection, migration and uptake of ethiodized oil in our experimental study are likely to be mainly by the lymphatic extracellular pathway. The injected ethiodized oil could slowly and gradually enter the initial lymphatics as small sized lipid droplets, and migrate into the sentinel node and be trapped within the node.
The nodal uptake of oil contrast media including ethiodized oil has been well known with direct lymphography using the direct intra-lymphatic injection of ethiodized oil (33, 34). Tanaka et al. (35) reported the incidental lymph node uptake of ethiodized oil after submucosal injection during CT examinations to evaluate the extent of esophageal cancer spread. Also, some previous reports (36, 37) demonstrated the regional lymph node uptake of ethiodized oil based contrast media with mural injection into the stomach of gastric cancer patients (36) and with submucosal injection into the rectum of healthy rabbits (37). However, these studies used simple radiography instead of CT examination. Moreover, although Karanov's (36) report demonstrated the uptake of ethiodized oil in almost all regional lymph nodes in patients with gastric cancer, this study was not inteneded to detect sentinel nodes, and the ethiodized oil emulsion was injected into predefined 20 points of the stomach.
After direct lymphography using oil contrast media, the disruption of the lymph node architecture followed by inflammatory reaction can take place in the lymph node. Even on the next day of injection, a marked degree of architectural distortion can be noted with disruption of the subcapsular sinusoidal spaces by large lipid vacuoles (38). However, in our study, there were small lipid droplets mainly in the subcapsular sinusoids and some of the medullary sinuses without architectural distortion in the lymph node. Lymph nodes have afferent lymphatic vessels converging into the cortical surface of the lymph node and efferent lymphatic vessels, leaving the lymph node from the medullary sinus (39). Thus, lipid droplets with Oil-red-O stain in the subcapsular sinusoids may reflect the drainage by afferent lymphatics from interstitial space in our study.
One of the limitations of our study is that the CT lymphography protocol may not have been fully optimized. We used post-1h and post-2h CT scans based on the result of our pilot study using two rabbits. However, a previous report showed that the degree of ethiodized oil retention in popliteal lymph nodes gradually increased even 1 week after injection without washout when ethiodized oil was injected in the hind paws of rats (40). Further delayed CT scan after 2 hours might have increased the sentinel node detection rate in our study. However, the sentinel node detection rate at post-2h CT was already high at 96% (50 of 52 blue stained nodes), and there were no cases in which CT lymphography failed to identify a sentinel node. Hence, we believe that the 2-hour delayed CT scan sufficiently serve its role for sentinel node detection.
In conclusion, CT lymphography using ethiodized oil may be feasible for sentinel node mapping in experimentally induced VX2 carcinoma in the rabbit thigh, which has an advantage of longer time window for node detection compared to water soluble contrast agents. Therefore, CT lymphography using ethiodized oil may be a promising technique for sentinel node mapping of soft tissue tumors.
Figures and Tables
Fig. 1
CT lymphography after ethiodized oil injection at peritumoral area in rabbit thigh.
A, B. Noncontrast CT images obtained 1 (A) and 2 (B) hours after ethiodized oil injection show nodular high attenuation areas (arrows), suggesting ethiodized oil retention in right femoroiliac lymph nodes. C. Photography obtained after resection of blue-stained lymphatic basin shows blue-stained lymph nodes (arrows). D. Specimen radiography shows ethiodized oil retention (arrows) in blue-stained lymph nodes. E. Photomicrography of removed node demonstrates intranodal lipid droplets (red droplets, arrows), indicating intra-nodal ethiodized oil retention (Oil-Red-O stain; original magnification, × 40).
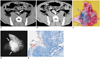
Fig. 2
Box-and-whisker plots of measured nodal attenuation values in Hounsfield unit (HU) at noncontrast CT before (pre-injection CT), 1 hour (post-1h CT) and 2 hours (post-2h CT) after ethiodized oil injection. Differences of attenuation values between all three pairs of pre- and post-1h and post-2h CT images were statistically significant (p < 0.0005, respectively).

References
1. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992; 127:392–399.
2. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997; 349:1864–1867.
3. Borgstein PJ, Pijpers R, Comans EF, van Diest PJ, Boom RP, Meijer S. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998; 186:275–283.
4. De Cicco C, Cremonesi M, Luini A, Bartolomei M, Grana C, Prisco G, et al. Lymphoscintigraphy and radioguided biopsy of the sentinel axillary node in breast cancer. J Nucl Med. 1998; 39:2080–2084.
5. Linehan DC, Hill AD, Akhurst T, Yeung H, Yeh SD, Tran KN, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimize sentinel node identification in breast cancer patients. Ann Surg Oncol. 1999; 6:450–454.
6. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006; 355:1307–1317.
7. Park DJ, Lee HJ, Lee HS, Kim WH, Kim HH, Lee KU, et al. Sentinel node biopsy for cT1 and cT2a gastric cancer. Eur J Surg Oncol. 2006; 32:48–54.
8. Suga K, Ogasawara N, Okada M, Matsunaga N. Interstitial CT lymphography-guided localization of breast sentinel lymph node: preliminary results. Surgery. 2003; 133:170–179.
9. Minato M, Hirose C, Sasa M, Nishitani H, Hirose Y, Morimoto T. 3-dimensional computed tomography lymphography-guided identification of sentinel lymph nodes in breast cancer patients using subcutaneous injection of nonionic contrast medium: a clinical trial. J Comput Assist Tomogr. 2004; 28:46–51.
10. Suga K, Yuan Y, Okada M, Matsunaga N, Tangoku A, Yamamoto S, et al. Breast sentinel lymph node mapping at CT lymphography with iopamidol: preliminary experience. Radiology. 2004; 230:543–552.
11. Tangoku A, Yamamoto S, Suga K, Ueda K, Nagashima Y, Hida M, et al. Sentinel lymph node biopsy using computed tomography-lymphography in patients with breast cancer. Surgery. 2004; 135:258–265.
12. Suga K, Shimizu K, Kawakami Y, Tangoku A, Zaki M, Matsunaga N, et al. Lymphatic drainage from esophagogastric tract: feasibility of endoscopic CT lymphography for direct visualization of pathways. Radiology. 2005; 237:952–960.
13. Suga K, Yamamoto S, Tangoku A, Oka M, Kawakami Y, Matsunaga N. Breast sentinel lymph node navigation with three-dimensional interstitial multidetector-row computed tomographic lymphography. Invest Radiol. 2005; 40:336–342.
14. Kim YH, Lee YJ, Park JH, Lee KH, Lee HS, Park YS, et al. Early gastric cancer: feasibility of CT lymphography with ethiodized oil for sentinel node mapping. Radiology. 2013; 267:414–421.
15. Handal JA, Schulz JF, Florez GB, Kwok SC, Khurana JS, Samuel SP. Creation of rabbit bone and soft tissue tumor using cultured VX2 cells. J Surg Res. 2013; 179:e127–e132.
16. Lei Z, Ma H, Xu N, Xi H. The evaluation of anti-angiogenic treatment effects for implanted rabbit VX2 breast tumors using functional multi-slice spiral computed tomography (f-MSCT). Eur J Radiol. 2011; 78:277–281.
17. Kim YH, Choi BI, Cho WH, Lim S, Moon WK, Han JK, et al. Dynamic contrast-enhanced MR imaging of VX2 carcinomas after X-irradiation in rabbits: comparison of gadopentetate dimeglumine and a macromolecular contrast agent. Invest Radiol. 2003; 38:539–549.
18. Sutton R, Tsopelas C, Kollias J, Chatterton BE, Coventry BJ. Sentinel node biopsy and lymphoscintigraphy with a technetium 99m labeled blue dye in a rabbit model. Surgery. 2002; 131:44–49.
19. Wu H, Ying H, Xi X, Shen N, Shu Y, Hoffman MR, et al. Localization of the sentinel lymph node in tongue VX2 carcinoma via indirect CT lymphography combined with methylene blue dye injection. Acta Otolaryngol. 2010; 130:503–510.
20. Daigeler A, Kuhnen C, Moritz R, Stricker I, Goertz O, Tilkorn D, et al. Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg. 2009; 394:321–329.
21. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993; 217:72–77.
22. Behranwala KA, A'Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Ann Surg Oncol. 2004; 11:714–719.
23. Sawamura C, Matsumoto S, Shimoji T, Ae K, Okawa A. Lymphadenectomy and histologic subtype affect overall survival of soft tissue sarcoma patients with nodal metastases. Clin Orthop Relat Res. 2013; 471:926–931.
24. Andreou D, Boldt H, Werner M, Hamann C, Pink D, Tunn PU. Sentinel node biopsy in soft tissue sarcoma subtypes with a high propensity for regional lymphatic spread--results of a large prospective trial. Ann Oncol. 2013; 24:1400–1405.
25. Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000; 18:2553–2559.
26. Feldman SM, Krag DN, McNally RK, Moor BB, Weaver DL, Klein P. Limitation in gamma probe localization of the sentinel node in breast cancer patients with large excisional biopsy. J Am Coll Surg. 1999; 188:248–254.
27. Dupont E, Cox C, Shivers S, Salud C, Nguyen K, Cantor A, et al. Learning curves and breast cancer lymphatic mapping: institutional volume index. J Surg Res. 2001; 97:92–96.
28. Mariani G, Moresco L, Viale G, Villa G, Bagnasco M, Canavese G, et al. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001; 42:1198–1215.
29. Tangoku A, Seike J, Nakano K, Nagao T, Honda J, Yoshida T, et al. Current status of sentinel lymph node navigation surgery in breast and gastrointestinal tract. J Med Invest. 2007; 54:1–18.
30. Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990; 70:987–1028.
31. Ikomi F, Hanna GK, Schmid-Schönbein GW. Mechanism of colloidal particle uptake into the lymphatic system: basic study with percutaneous lymphography. Radiology. 1995; 196:107–113.
32. Kan Z, McCuskey PA, Wright KC, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994; 29:990–993.
33. Davidson JW, Fletch AL, McIlmoyle G, Roeck W. The technique and applications of lymphography. Can J Comp Med. 1973; 37:130–138.
34. Guermazi A, Brice P, Hennequin C, Sarfati E. Lymphography: an old technique retains its usefulness. Radiographics. 2003; 23:1541–1558. discussion 1559-1560.
35. Tanaka Y, Sawada S, Yoshida M, Murata T. Clinical evaluation of computed tomography images in esophageal cancer after submucosal injection of iodized oil. J Comput Tomogr. 1985; 9:125–132.
36. Karanov SI. Endoscopic indirect lymphography in gastric cancer. Endoscopy. 1988; 20:241–243.
37. Dietrich A, Wuttke M, Walter F, De Leon I, Kloeppel R, Schoenfelder M. Indirect lymph node lymphangiography using an iodine-based contrast medium and projection radiography following submucosal injection in a rabbit model. Langenbecks Arch Surg. 2002; 387:315–319.
38. Kazem I, Nedwich A, Mortel R, Honda T. Comparative histological changes in the normal lymph node following ethiodol lymphography and colloidal gold-198 lymphscanning. Clin Radiol. 1971; 22:382–388.
39. Clément O, Luciani A. Imaging the lymphatic system: possibilities and clinical applications. Eur Radiol. 2004; 14:1498–1507.
40. Chung YE, Hyung WJ, Kweon S, Lim SJ, Choi J, Lee MH, et al. Feasibility of interstitial CT lymphography using optimized iodized oil emulsion in rats. Invest Radiol. 2010; 45:142–148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download