Abstract
We present a case of a patient with rapid deterioration of esophageal varices caused by portal hypertension accompanied by a large arterioportal shunt that developed after radiofrequency ablation of hepatocellular carcinoma. We used n-butyl cyanoacrylate (NBCA) as an embolic material to achieve pinpoint embolization of the shunt, because the microcatheter tip was 2 cm away from the shunt site. Under hepatic arterial flow control using a balloon catheter, the arterioportal shunt was successfully embolized with NBCA, which caused an improvement in the esophageal varices.
Although intrahepatic arterioportal shunts rarely develop after radiofrequency ablation (RFA) (1), they may cause portal hypertension and variceal bleeding (2). We describe a case of a patient with rapid deterioration of esophageal varices caused by a large arterioportal shunt that developed after RFA of hepatocellular carcinoma (HCC). The large arterioportal shunt was successfully embolized with n-butyl cyanoacrylate (NBCA) (3). Because it is difficult to determine the site and size of the shunt with angiography, we used volume-rendered images from computed tomography performed during hepatic arteriography. Additionally, because the blood flow through a large shunt is very fast, we used a balloon catheter to decrease the blood flow in the hepatic artery during embolization of the shunt (4). To the best of our knowledge, this is the first report describing the use of a balloon catheter for achieving blood flow control during embolization of an arterioportal shunt.
A 70-year-old male with liver cirrhosis caused by hepatitis C virus was being followed up at our institution. As contrast-enhanced computed tomography (CE-CT) revealed three HCC lesions of < 15 mm in diameter in segments 1, 2, and 4; RFA of each lesion was performed. However, esophageal varices rapidly deteriorated at 3 months after RFA. Although endoscopic injection sclerotherapy was performed, the esophageal varices deteriorated further, and endoscopy showed greatly enlarged varices (F3) that were red in color (Fig. 1A). According to Idezuki (5), varices can be classified into three types based on their size: F1 varices are straight small caliber varices; F2 varices are larger with a bead-like appearance; and F3 varices are the largest, with a nodular or tumor-like shape. Red color signs indicate a high risk of bleeding from varices. Arterial phase CE-CT images revealed a marked arterioportal shunt in the left lateral segment of the liver (Fig. 1B) and portal thrombosis. Therefore, embolization of the arterioportal shunt was planned.
Angiography of the common hepatic artery before embolization revealed a large arterioportal shunt, but the precise site and size of the shunt were unclear (Fig. 1C). Volume-rendered images from CT during angiography revealed the precise site and size (5 mm) of the arterioportal shunt (Fig. 1D). A 5.2-Fr balloon catheter (Selecon MP Catheter II, Cobra type; Terumo Clinical Supply, Gifu, Japan) was inserted into the proper hepatic artery via the right femoral artery. A 2-Fr microcatheter (Cerisier; Medikit, Tokyo, Japan) was then advanced as close as possible to the shunt site. NBCA was mixed with iodized oil (lipiodol) at a ratio of 1:2 to control its polymerization time and to make it radiopaque. The NBCA mixture (0.5 mL) was infused through the microcatheter during flow control in the hepatic artery with a balloon catheter. The hepatic artery was incompletely occluded using a balloon to maintain antegrade blood flow in the hepatic artery (Fig. 1E). A small amount of the NBCA mixture leaked into a portal vein during embolization. The arterioportal shunt had completely disappeared after embolization, and the NBCA mixture was found at the shunt site (Fig. 1F). Arterial phase CE-CT images obtained after embolization confirmed that the arterioportal shunt had disappeared (Fig. 1G).
After embolization of the arterioportal shunt, the esophageal varices decreased in size from F3 to F1. However, the varices were still red in color. Therefore, endoscopic injection sclerotherapy was performed again. Endoscopy performed at 4 months after embolization confirmed that the varices had decreased in size from F3 to F1, and the red color had disappeared (Fig. 1H). CE-CT performed at 6 months after embolization showed no evidence of recurrence of an arterioportal shunt, the presence of NBCA mixture at the shunt site in segment 3 of the liver and in the left portal vein, and necrotic lesions caused by RFA (Fig. 1I, J).
Arterioportal shunt is a relatively rare complication of RFA. Nine arterioportal shunts were reported in 2320 patients (3554 lesions) (1). However, a large arterioportal shunt causes portal hypertension, resulting in the worsening and rupture of esophageal varices (2). Therefore, it is essential to treat large arterioportal shunts. As surgical operation is often too invasive (6), non-invasive procedures, such as transcatheter arterial embolization (TAE), are generally preferred.
There are several problems associated with embolization of a large arterioportal shunt. On angiography, the precise site and size of the shunt are usually unclear, and blood flow through large shunts may be too fast, necessitating interventions to decrease the blood flow during embolization to prevent leakage of the embolic material into the portal vein. In our patient, we used volume-rendered images from CT performed during hepatic arteriography to evaluate the precise site and size of the shunt. Using these images, we were able to observe the arterioportal shunt from any angle, and visualize the entire vascular tree. We also used a balloon catheter to control the blood flow through the shunt (4). To the best of our knowledge, there are no other reports describing the use of balloon catheters to control blood flow during embolization of an arterioportal shunt. The balloon was used to incompletely occlude the hepatic artery and to maintain antegrade blood flow in the hepatic artery. If the artery is completely occluded, retrograde blood flow causes reflux of the NBCA mixture, resulting in attachment of the NBCA mixture to the microcatheter.
Embolic materials (7) that can be used in TAE of an arterioportal shunt include metallic coils (8), NBCA mixtures, alcohol, gelatin sponges or Ivalon particles, and detachable balloons (9). We used a concentrated NBCA mixture (NBCA:lipiodol = 1:2) in this patient to achieve pinpoint embolization of the shunt. It is possible to control the polymerization time of the NBCA mixture by adjusting the NBCA:lipiodol ratio. Pollak and White (10) reported an in vivo polymerization time of 1-4 second for NBCA:lipiodol ratios ranging from 1:1 to 1:4, and found a linear relationship between the ratio and polymerization time. Based on this result, we considered that an NBCA mixture was the best embolic material for use in our patient. As a small amount of the NBCA mixture leaked into the portal vein, the use of a more concentrated NBCA mixture with a shorter polymerization time (e.g., NBCA:lipiodol = 1:1) was necessary and it could have avoided this problem. Although other options were considered, they were not deemed appropriate. In this case, treatment with microcoils would have resulted in proximal embolization, because the microcatheter tip was 2 cm away from the shunt site. This would cause retrograde blood flow through peripheral collaterals, and result in shunt embolization failure. Alcohol would also have been risky because it is not a radiopaque agent and therefore cannot be seen under fluoroscopy. Gelatin sponges or Ivalon particles pass through a shunt very easily. Finally, it is impossible to insert a detachable balloon into a thin artery.
In conclusion, volume-rendered images are very helpful for demonstrating the precise site and size of an arterioportal shunt. A balloon catheter can be used to control the blood flow and to perform embolization of a large arterioportal shunt with the NBCA mixture injected through a 2-Fr microcatheter that was advanced as close as possible to the shunt site. This embolization procedure is relatively noninvasive compared with surgical methods.
Figures and Tables
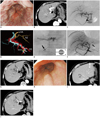
Fig. 1
N-butyl cyanoacrylate (NBCA) embolization of arterioportal shunt under blood flow control with balloon catheter.
A. Endoscopy before embolization revealed large red-colored esophageal varices. B. Arterial phase contrast-enhanced computed tomography performed before embolization revealed early enhancement of left portal vein (arrow), suggesting presence of arterioportal shunt. C. Angiography performed before embolization revealed presence of large arterioportal shunt (arrow) in left lateral segment of liver. D. Volume-rendered images from computed tomography performed during hepatic arteriography. Superior view showed that shunt site (yellow) was 5 mm in diameter (arrow), and was located between hepatic artery (red) and portal vein (light blue) in segment 3 of liver. E. NBCA mixture was injected through 2-Fr microcatheter that was advanced as close as possible to arterioportal shunt under blood flow control using balloon catheter (arrow) that was inserted in proper hepatic artery. Balloon was used to incompletely occlude artery, allowing antegrade blood flow (inset at bottom right). F. Angiography performed after embolization showed complete disappearance of shunt and presence of NBCA mixture at shunt site (arrow). LHA = left hepatic artery. G. Contrast-enhanced computed tomography (CE-CT) after embolization showed complete disappearance of shunt. H. Endoscopy performed at 6 months after embolization confirmed that varices had shrunk and red color sign had disappeared. I, J. CE-CT performed at 6 months after embolization revealed presence of NBCA mixture at shunt site in segment 3 of liver, and in left portal vein (arrows). Necrotic lesions (asterisks) caused by radiofrequency ablation were found in segments 8 and 4 of liver.
References
1. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003; 226:441–451.
2. Lazaridis KN, Kamath PS. Images in hepatology. Arterioportal fistula causing recurrent variceal bleeding. J Hepatol. 1998; 29:142.
3. Kerlan RK Jr, Bank WO, Hoddick WK, Pogany AC, Sollenberger RD. Occlusion of a hepatic artery to portal vein fistula with bucrylate. Cardiovasc Intervent Radiol. 1983; 6:138–140.
4. Kanematsu M, Kondo H, Goshima S, Tsuge Y, Watanabe H, Moriyama N. Giant high-flow type pulmonary arteriovenous malformation: coil embolization with flow control by balloon occlusion and an anchored detachable coil. Korean J Radiol. 2012; 13:111–114.
5. Idezuki Y. Japanese Society for Portal Hypertension. General rules for recording endoscopic findings of esophagogastric varices (1991). World J Surg. 1995; 19:420–422. discussion 423.
6. Strodel WE, Eckhauser FE, Lemmer JH, Whitehouse WM Jr, Williams DM. Presentation and perioperative management of arterioportal fistulas. Arch Surg. 1987; 122:563–571.
7. Chen Q, Tack C, Morcos M, Ruggiero M, Schlossberg P, Fogel J, et al. Embolotherapy of an arterioportal fistula. Cardiovasc Intervent Radiol. 2007; 30:1047–1051.
8. Applbaum YN, Renner JW. Steel coil embolization of hepatoportal fistulae. Cardiovasc Intervent Radiol. 1987; 10:75–79.
9. Orons PD, Zajko AB, Jungrels CA. Arterioportal fistula causing portal hypertension and variceal bleeding: treatment with a detachable balloon. J Vasc Interv Radiol. 1994; 5:373–376.
10. Pollak JS, White RI Jr. The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001; 12:907–913.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download