Abstract
We report a case of a 74-year-old female with myxoid adrenocortical adenoma which showed different magnetic resonance imaging findings compared to those of a typical adrenocortical adenoma. The myxoid change in the adrenocortical adenoma is a rare form of degeneration. It presents a considerable diagnostic challenge to both radiologists and clinicians because it can mimic other adrenal tumor types on imaging. The MRI findings of the presented case included a high signal intensity on T2-weighted images similar to that of fluid and delayed progressive enhancement.
An adrenocortical adenoma is a benign neoplasm which is derived from cells of the adrenal cortex and may be functionally active or nonfunctional. The discrimination of adrenocortical adenoma from nonadenoma is a common problem for radiologists in the daily practice.
Computed tomography (CT) and magnetic resonance (MR) imaging have been found to be useful imaging modalities for the differentiation between adenomas and nonadenomas. Unenhanced CT and chemical-shift MR imaging are based on identifying intracytoplasmic lipids in the adrenocortical adenoma. Whereas a delayed contrast-enhanced CT performed 15 minutes after contrast material injection is based on the different contrast washout characteristics of adenoma and nonadenoma (1, 2).
Recently we encountered a myxoid adrenocortical adenoma in which the imaging findings were inconsistent with typical MR imaging findings of an adrenocortical adenoma. The neoplasm is exceedingly rare, with fewer than 60 case reports since the first description of this entity in 1979 (3). The most previous reports were concentrated on the pathological features of myxoid adrenocortical adenoma. To date, there have been no radiological reports concerning the radiologic features of myxoid adrenocortical adenoma. Hence, we present the imaging findings of a myxoid adrenocortical adenoma with an emphasis on MR imaging features and we will discuss the differential diagnoses.
A 74-year-old female was admitted to our hospital for an evaluation of diffuse abdominal pain. She had a history of hypertension with antihypertensive treatment medication. The results of a physical examination were unremarkable. Laboratory tests, including a full blood count, urea and electrolyte measurements, were within normal ranges.
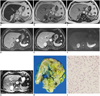
A contrast-enhanced CT scan of the abdomen during the portal venous phase demonstrated a well-circumscribed, 6-cm, ovoid-shaped, heterogeneously enhancing mass in the right adrenal gland. Thereafter a MR imaging was performed and a smooth, ovoid-shaped mass in the right adrenal gland with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images similar to that of fluid was revealed (Fig. 1A). Signal loss on out-of-phase images compared with that on in-phase images were not observed in the tumor (Fig. 1B, C). The enhancement pattern was early heterogeneous and showed a delayed progressive enhancement (Fig. 1D, E). The mass showed hyperintensity on high b-value (1000 s/mm2) diffusion weighted MR images, but the corresponding high apparent diffusion coefficient value confirmed that the hyperintensity on diffusion-weighted MR images represented T2 shine-through rather than restricted diffusion (Fig. 1F, G).
The serum and urine marker levels, including those of metanephrines, cortisol, aldosterone and catecholamine, as well as the results of the 24-hour urine collection for vanillylmandelic acid for the detection of a hormonally active tumor were in the normal range.
The patient underwent a right adrenalectomy in the operating room.
On the cut section, the tumor measured 6.0 × 4.7 × 1.6 cm, was well-circumscribed, a yellow-greenish mass and had a gelatinous consistency (Fig. 1H). Microscopically, more than 90% of the tumor mass were characterized by a myxoid background containing anastomosing cords and tubules of tumor cells (Fig. 1I). Most of the tumor cells had a uniform polygonal shape with a distinct cell border. Neither mitotic figures nor necrosis were observed as well as no evidence of vascular or capsular invasion. The final pathologic diagnosis was myxoid adrenocortical adenoma.
Myxoid change is a nonspecific but not uncommon phenomenon in soft tissue tumors (4). A significant myxoid background has been incorporated into officially sanctioned nosologic classification, such as myxoma. In addition, a series of tumors may show myxoid changes of the stroma-e.g., myxoid degeneration in a leiomyoma.
However, myxoid adrenocortical tumors are an extremely rare morphologic variant of adrenocortical neoplasm (5). To our knowledge, only 56 cases have been reported to date: 23 myxoid adrenocortical adenomas, 2 borderline myxoid adrenocortical neoplasms and 31 myxoid adrenocortical carcinomas. The first case was described by Tang et al. (3) in 1979 and was diagnosed as a myxoid adrenocortical carcinoma. Brown et al. (6) reviewed 300 adrenocortical neoplasms from the Mayo Clinic from 1965 to 1984 and reported that less than 3% of the carcinomas and 1% of the adenomas had myxoid components. A characteristic feature of these tumors is a myxoid stroma containing cords and nests of cells, some of which exhibit lumens imparting a pseudoglandular appearance (6-9). The extent of the myxoid change in the reported cases is variable between 10% and more than 90%. The pathogenesis of the myxoid change has not been established yet. It has been thought to be induced by degenerative changes or due to the production of the tumor cells themselves (5, 7). However, the latter is unlikely because myxoid material was not demonstrated in the cytoplasm of tumor cells in the previously reported cases (6, 8) as well as in our case.
Several previous reports have been identified by us in the Anglophone literature concerning myxoid adrenocortical adenoma. But these focused on pathological and not on radiological features. To our knowledge, the presented case is the first description of a radiologic finding of myxoid adrenocortical adenoma. An evaluation was not possible whether a distinction exists between the CT findings of the myxoid variant and a normal adrenal adenoma because neither an unenhanced CT nor a 10 or 15 minutes delayed contrast-enhanced CT were performed. As we mentioned above, the MR findings in the presented case were different from typical adrenal adenoma MR findings. It might be that the differences in the MR findings may be attributed to the myxoid changes because the aforementioned imaging features of the tumor were similar to those of other myxoid-containing soft tissue tumors.
Myxoid material is composed of a gelatinous matrix stroma with high levels of hyaluronic acid and immature collagen fibers (10). Because of its high water content, myxoid material appears hyperintense on T2-weighted MR images. Additionally, a delayed progressive enhancement may correlate with an abundant myxoid matrix because the enlarged extracellular space in myxoid tissue results in greater accumulation of the contrast medium in the late phase (11). Thus, in our case seemed the high signal intensity on T2-weighted images and the delayed progressive enhancement of the tumor to be related to the myxoid components. Chemical shift imaging is an MR imaging technique used to detect microscopic fat within an organ and is the most sensitive method of differentiating adenomas from other tumors of the adrenal gland (12). Microscopic amounts of fat can be detected by the observation of signal intensity loss on out-of-phase images. As demonstrated in our case, the large amount of myxoid matrix may possibly contribute to the lack of this characteristic feature on chemical shifting. With this radiologic findings the diagnostic considerations should include pheochromocytoma, ganglioneuroma, schwannoma and hemangioma. Although the light bulb sign (classic, very high signal on T2-weighted images) is neither specific nor sensitive (13), an adrenal mass with very high signal on T2-weighted images, as seen in our case, leads usually to the more likely diagnosis pheochromocytoma. Additionally, pheochromocytomas are lipid-poor and will not decrease in signal intensity during out-of-phase sequences. However, pheochromocytomas have been described to be avidly enhanced on T1-weighted imaging after the administration of gadolinium-based contrast material. With this the capillary-rich framework of the tumor with a persistent enhancement on the delayed phase will be reflected (14) and thus, a delayed progressive enhancement is unlikely. Furthermore, the clinical presentation, specific biochemical tests and functional imaging can be helpful in diagnosing pheochromocytoma.
Because of its similar imaging characteristics to those of myxoid adrenocortical adenoma, ganglioneuroma is one of the main differential diagnoses. Histologically, ganglioneuromas show abundant myxoid matrices and a relatively small number of ganglion cells (15, 16). Their T2 signal intensity depends on the proportion of myxoid stroma to cellular components and the amount of collagen fibers in the tumor. If the proportion of myxoid stroma is high, the signal intensity of the tumor on T2-weight images will be high also. Thus, if a lesion contains a large amount of myxoid stroma it is expected to have a markedly high signal intensity on T2-weighted images. Ichikawa et al. (17) reported that the enhancement pattern of ganglioneuroma presented a delayed progressive uptake. This might be explained by the presence of an abundance of myxoid matrices in the tumors, as shown in our case. A whorled appearance, one of the MR imaging characteristics of ganglioneuroma, was indicated by curvilinear bands of low signal intensity on T2-weighted images corresponding to interlacing bundles of Schwann cells and collagen fibers within the tumor. This finding may be helpful in the diagnosis of ganglioneuroma.
Although it is extremely rare, adrenal schwannoma and juxta-adrenal schwannoma should be considered also in the differential diagnosis. Schwannomas have been described with not only a high intensity on T2-weighted images but also with an early mild enhancement and a progressive enhancement, similar to our case (18). Imaging findings of adrenal schwannoma have been reported to be similar to those of retroperitoneal schwannomas like a juxta-adrenal schwannoma (19). Additionally, due to its proximity to the adrenal gland there have been many case reports published of juxta-adrenal schwannoma simulating adrenal tumors. Hence, juxta-adrenal schwannoma and adrenal schwannoma are entities to be considered in the differential diagnosis. A schwannoma that grows to a large size commonly undergoes degenerative changes, such as cyst formation, calcification and hemorrhage (20). These findings can help to diagnose more specific. Another extremely rare benign tumor, an adrenal hemangioma, may show similar imaging findings to those in our case also. The presence of calcification is relatively common but doesn't always indicate an adrenal hemangioma (21). On T2-weighted images, the signal is markedly hyperintense. A characteristic imaging feature is the peripheral enhancement which persists in delayed images. However, due to the more prominent necrotic and fibrotic areas the delayed central filling is not as common as in hepatic hemangiomas. It might be helpful to differentiate it from other adrenal masses (22).
In summary, we have presented a pathological confirmed case of myxoid adrenocortical adenoma depicted on MR imaging. The imaging diagnosis of myxoid adrenocortical adenoma is challenging. The awareness of the entity and its imaging findings might be helpful to avoid a misdiagnosis, although the imaging findings are substantial overlapping with various other adrenal masses and a myxoid adrenocortical adenoma is a rare disease.
Figures and Tables
Fig. 1
MR and pathologic findings of myxoid adrenocortical adenoma in 74-year-old woman.
A. Axial T2-weighted MR imaging shows high signal intensity similar to that of fluid in tumor. B, C. Axial in-phase (B) and out-of-phase (C) gradient-echo T1-weighted images demonstrate lack of signal intensity loss on out-of-phase images compared with in-phase images in tumor. D, E. Enhancement pattern of tumor on T1-weighted volumetric interpolated breath-hold examination sequence in arterial (D) and delayed phases (E) represents early heterogeneous enhancement and progressive filling. F. Axial diffusion-weighted imaging (b = 1000 s/mm2) demonstrates high signal intensity in adrenal mass. G. Axial ADC map helps to confirm presence of T2 shine-through, showing high signal intensity in adrenal mass. H. On cut section of gross specimen, tumor shows well-circumscribed, yellow-greenish and glistening myxoid appearance. I. Lower magnification of photomicrograph (hematoxylin-eosin stain; original magnification × 40) shows tumor cells arranged in cords and tubules with large amounts of extra-cellular myxoid material.
References
1. Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000; 217:798–802.
2. Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004; 231:711–716.
3. Tang CK, Harriman BB, Toker C. Myxoid adrenal cortical carcinoma: a light and electron microscopic study. Arch Pathol Lab Med. 1979; 103:635–638.
4. Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000; 4:99–123.
5. Forsthoefel KF. Myxoid adrenal cortical carcinoma. A case report with differential diagnostic considerations. Arch Pathol Lab Med. 1994; 118:1151–1153.
6. Brown FM, Gaffey TA, Wold LE, Lloyd RV. Myxoid neoplasms of the adrenal cortex: a rare histologic variant. Am J Surg Pathol. 2000; 24:396–401.
7. Honda K, Kashima K, Daa T, Gamachi A, Nakayama I, Yokoyama S. Myxoid adrenal cortical adenoma. Pathol Int. 2001; 51:887–891.
8. Dundr P, Novák K. Pseudoglandular myxoid adenoma of the adrenal gland. Pathol Res Pract. 2003; 199:493–496.
9. Bollito ER, Papotti M, Porpiglia F, Terzolo M, Cracco CM, Cappia S, et al. Myxoid adrenocortical adenoma with a pseudoglandular pattern. Virchows Arch. 2004; 445:414–418.
10. Wu JS, Hochman MG. Soft-tissue tumors and tumorlike lesions: a systematic imaging approach. Radiology. 2009; 253:297–316.
11. Bydder GM. Clinical application of gadolinium-DTPA. In : Stark DD, Bradley WG, editors. Magnetic resonance imaging. St. Louis: Mosby;1988. p. 182–200.
12. Mitchell DG, Crovello M, Matteucci T, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology. 1992; 185:345–351.
13. Varghese JC, Hahn PF, Papanicolaou N, Mayo-Smith WW, Gaa JA, Lee MJ. MR differentiation of phaeochromocytoma from other adrenal lesions based on qualitative analysis of T2 relaxation times. Clin Radiol. 1997; 52:603–606.
14. Inan N, Arslan A, Akansel G, Anik Y, Balci NC, Demirci A. Dynamic contrast enhanced MRI in the differential diagnosis of adrenal adenomas and malignant adrenal masses. Eur J Radiol. 2008; 65:154–162.
15. Sakai F, Sone S, Kiyono K, Maruyama A, Ueda H, Aoki J, et al. Intrathoracic neurogenic tumors: MR-pathologic correlation. AJR Am J Roentgenol. 1992; 159:279–283.
16. Dunnick NR, Castellino RA. Arteriographic Manifestations of Ganglioneuromas. Radiology. 1975; 115:323–328.
17. Ichikawa T, Ohtomo K, Araki T, Fujimoto H, Nemoto K, Nanbu A, et al. Ganglioneuroma: computed tomography and magnetic resonance features. Br J Radiol. 1996; 69:114–121.
18. Liu QY, Gao M, Li HG, Lin XF, Huang SQ, Liang BL. Juxta-adrenal schwannoma: dynamic multi-slice CT and MRI findings. Eur J Radiol. 2012; 81:794–799.
19. Suzuki K, Nakanishi A, Kurosaki Y, Nogaki J, Takaba E. Adrenal schwannoma: CT and MRI findings. Radiat Med. 2007; 25:299–302.
20. Takatera H, Takiuchi H, Namiki M, Takaha M, Ohnishi S, Sonoda T. Retroperitoneal schwannoma. Urology. 1986; 28:529–531.
21. Sabanegh E Jr, Harris MJ, Grider D. Cavernous adrenal hemangioma. Urology. 1993; 42:327–330.
22. Otal P, Escourrou G, Mazerolles C, Janne d'Othee B, Mezghani S, Musso S, et al. Imaging features of uncommon adrenal masses with histopathologic correlation. Radiographics. 1999; 19:569–581.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download