Abstract
Superior vena cava (SVC) obstruction is associated with the gradual development of venous collaterals. We present a rare form of systemic-to-pulmonary subpleural collateral pathway that developed in the bridging subpleural pulmonary veins in a 54-year-old woman with complete SVC obstruction. This uncommon collateral pathway represents a rare form of acquired right-to-left shunt due to previous pleural adhesions with an increased risk of stroke due to right-to-left venous shunting, which requires lifelong anticoagulation.
Superior vena cava (SVC) syndrome can lead to the formation of venous collaterals, depending on the severity of the occlusion and the time span since its development (1). The clinical presentation of SVC occlusion may be delayed, if the occlusion occurs gradually and allows adequate time for the development of collateral veins. Systemic-to-pulmonary subpleural venous collateral pathway is an uncommon collateral pathway and a rare form of acquired right-to-left shunt (2, 3) due to previous pleural adhesions with an increased risk of stroke due to right-to-left venous shunting, thus requiring lifelong anticoagulation (1, 2, 4, 5).
A 54-year-old woman presented with intermittent swelling of the face and arms. Her past surgical and medical history included pleurodesis for recurrent left-sided pleural effusions and Factor V Leiden deficiency disorder, for which she was taking warfarin. This was further complicated by recurrent hypercoagulable events including deep venous thrombosis and pulmonary embolisms. On examination, her body temperature was 36.8℃, heart rate was 94 beats per minute, blood pressure was 132/68 mm Hg, and respiratory rate was 18 breaths per minute. Clinical examination also revealed bilateral lung basal crackles, and prominent engorged vasculature in the neck and anterior chest wall.
Multidetector computed tomographic (MDCT) venography of the chest was performed. Coronal reformation images (Fig. 1A), maximum-intensity-projection images (Fig. 1B, C), and volume-rendered images (Fig. 1D) demonstrated complete chronic SVC obstruction (Fig. 1A) with numerous resultant paravertebral and chest wall venous collaterals (Fig. 1D), representing the involvement of lateral thoracic and paravertebral collateral pathways. In addition, a few patent left-sided pleuro-pulmonary venous collaterals (Fig. 1B-D) extending from the chest wall, crossing the pleura and lingular lobe, and finally draining into the left superior pulmonary vein were seen, and this finding was consistent with the presence of systemic-to-pulmonary venous collateral pathway (SPVC) and bridging subpleural pulmonary veins.
Since our patient had complete chronic SVC occlusion resulting in the formation of right-to-left systemic-to-pulmonary venous collaterals, a coexisting hypercoagulable state, and deep vein thrombosis, subsequent lifelong anticoagulation with warfarin was initiated to reduce the risk of stroke. Angioplasty or stenting of the SVC was not recommended, because the SVC occlusion was secondary to a hypercoagulable state, rather than due to external compression, which in most cases, is due to malignancy. Due to the presence of extensive collaterals, embolization of the venous collaterals was not performed, and the patient was discharged after symptomatic improvement.
This is an extremely rare case of SVC syndrome due to a hypercoagulable state presenting with a right-to-left extracardiac shunt through bridging pleuro-pulmonary venous collaterals demonstrated on MDCT. Malignancy is the most common cause of SVC syndrome, and it accounts for two-thirds of the cases, while the remaining one-third of the cases have a benign etiology. SVC obstruction due to benign causes is most commonly due to indwelling large-bore venous catheters or pacer wires, while the other benign causes include fibrosing mediastinitis, aortic aneurysm, or infectious diseases (3, 6). However, regardless of its cause, SVC syndrome develops secondary to the obstruction of the SVC or brachiocephalic veins, resulting in venous congestion; thus leading to the formation of prominent venous collaterals to facilitate drainage of the venous blood to the heart (1). Commonly, SVC syndrome presents as facial edema, as in our patient, along with lightheadedness, engorged superficial head and neck veins, and orthopnea; while neurologic symptoms and laryngeal edema can occur in cases with severe SVC obstruction (1, 7).
Multidetector computed tomography is particularly beneficial in the diagnosis of SVC obstruction and SPVC (1, 2, 6). MDCT is less invasive, and it is comparable or even superior to conventional venography in assessing the site of obstruction, collateral venous pathways and associated thoracic abnormalities (1). In addition to the routine two-dimensional axial images, maximum-intensity projection and 3-dimensional volume-rendering reconstructions of MDCT can accurately demonstrate the anatomy of the great vessels, presence of SPVC and tortuous draining veins in detail (1, 2). The presence of SVC occlusion with venous collaterals is a reliable criterion for diagnosing SVC syndrome on CT, which has a sensitivity and specificity of 96% and 92%, respectively (8, 9).
Four major thoracic venous collateral pathways have been reported in the literature, and they include the azygos-hemiazygos pathway, internal/external mammary pathway, lateral thoracic pathway, and paravertebral pathway (1, 2, 4, 5); while unusual pathways include SPVC and portocaval routes (4, 5). Among the SPVC pathways, the connection between the brachiocephalic vein and the superior pulmonary vein via the bronchial venous plexus is the commonly reported pathway (4). However, our case had an uncommon type of SPVC, in which these pleuro-pulmonary venous collaterals were developed due to previous left-sided pleurodesis that was performed for recurrent left-sided pleural effusions. Angiogenesis within the pleural adhesions between the chest wall and lungs has been postulated as an underlying mechanism for the formation of bridging subpleural pulmonary veins (2), which drain into the left superior pulmonary vein. Thus, our patient had an acquired type of SPVC, with pleural adhesions and chronic inflammatory processes being the major precipitating factors (4). Overall, SPVC can be clinically important as the venous collaterals bypass the pulmonary circulation and may potentially result in intracranial emboli and high cardiac output failure (4), which fortunately were not observed in our patient. This uncommon venous collateral pathway represents a rare form of acquired right-to-left shunt due to previous pleural adhesions and represents a potential risk factor for stroke due to right-to-left venous shunting, which requires lifelong anticoagulation due to the concomitant presence of factor V Leiden deficiency and SPVC.
Figures and Tables
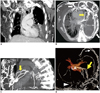 | Fig. 1Multidetector computed tomographic venography images of chest in 54-year-old woman with complete SVC obstruction and abundant venous collaterals.
A. Coronal reformatted image demonstrates complete chronic SVC obstruction with abundant adjacent mediastinal collateral venous drainage (white thin arrow). B-D. Axial (B) and oblique sagittal maximum-intensity-projection images (C) along with volume-rendered images (D) reveal multiple venous collaterals in chest wall (white bold arrows) and paravertebral regions (arrowhead), representing involvement of lateral thoracic and paravertebral collateral pathways. Note dense contrast in patent left-sided venous collaterals (yellow bold arrow, B-D) extending from chest wall, crossing pleura, subpleural pulmonary veins and lingular lobe before draining into left superior pulmonary vein, which is consistent with connections between lateral thoracic pathway and systemic-to-pulmonary venous collateral pathway and bridging subpleural pulmonary veins.
SVC = superior vena cava
|
References
1. Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease. Imaging with multi-detector row CT. Eur J Radiol. 2006; 59:93–103.
2. Kim HC, Chung JW, Yoon CJ, Lee W, Jae HJ, Kim YI, et al. Collateral pathways in thoracic central venous obstruction: three-dimensional display using direct spiral computed tomography venography. J Comput Assist Tomogr. 2004; 28:24–33.
3. Sze DY, Fleischmann D, Ma AO, Price EA, McConnell MV. Embolization of a symptomatic systemic to pulmonary (right-to-left) venous shunt caused by fibrosing mediastinitis and superior vena caval occlusion. J Vasc Interv Radiol. 2010; 21:140–143.
4. Kapur S, Paik E, Rezaei A, Vu DN. Where there is blood, there is a way: unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010; 30:67–78.
5. Kim HC, Chung JW, Park SH, Kim HB, Jae HJ, Lee W, et al. Systemic-to-pulmonary venous shunt in superior vena cava obstruction: depiction on computed tomography venography. Acta Radiol. 2004; 45:269–274.
6. Koetters KT. Superior vena cava syndrome. J Emerg Nurs. 2012; 38:135–138.
7. Lawler LP, Fishman EK. Thoracic venous anatomy multidetector row CT evaluation. Radiol Clin North Am. 2003; 41:545–560.
8. Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol. 2010; 194:W336–W346.
9. Kim HJ, Kim HS, Chung SH. CT diagnosis of superior vena cava syndrome: importance of collateral vessels. AJR Am J Roentgenol. 1993; 161:539–542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download