Abstract
Objective
The aim of this study was to determine the interobserver and intermodality agreement in the interpretation of time-of-flight (TOF) MR angiography (MRA) for the follow-up of coiled intracranial aneurysms with the Enterprise stent.
Materials and Methods
Two experienced neurointerventionists independently reviewed the follow-up MRA studies of 40 consecutive patients with 44 coiled aneurysms. All aneurysms were treated with assistance from the Enterprise stent and the radiologic follow-up intervals were greater than 6 months after the endovascular therapy. Digital subtraction angiography (DSA) served as the reference standard. The degree of aneurysm occlusion was determined by an evaluation of the maximal intensity projection (MIP) and source images (SI) of the TOF MRA. The capability of the TOF MRA to depict the residual flow within the coiled aneurysms and the stented parent arteries was compared with that of the DSA.
Results
DSA showed stable occlusions in 25 aneurysms, minor recanalization in 8, and major recanalization in 11. Comparisons between the TOF MRA and conventional angiography showed that the MIP plus SI had almost perfect agreement (κ = 0.892, range 0.767 to 1.000) and had better agreement than with the MIP images only (κ = 0.598, range 0.370 to 0.826). In-stent stenosis of more than 33% was observed in 5 cases. Both MIP and SI of the MRA showed poor depiction of in-stent stenosis compared with the DSA.
Since the International Subarachnoid Aneurysm Trial, endovascular coil embolization has been widely used for the treatment of intracranial aneurysms (1, 2). Moreover, improved devices and advanced coiling techniques have made it possible to treat an increased number of aneurysms, including those with difficult configurations. Among them, the stent protection technique has significantly widened the applicability of endovascular therapy (3). However, it is relatively well known that coiled aneurysms have a relatively high recanalization rate. Although digital subtraction angiography (DSA) is considered to be a standard test to assess recanalization, there are many reports that MR angiography (MRA), which is noninvasive and non-irradiating in nature, is useful as a follow-up tool in coiled aneurysms (2, 4-6). In patients treated with stent-assisted coil embolizations, however, there have been concerns of a susceptibility artifact or radiofrequency shielding by a closed-cell stent, especially with the Enterprise (Codman, Raynham, MA, USA). Therefore, we evaluated the interobserver and intermodality agreement in the interpretation of the time of flight (TOF) MRA for the follow-up of coiled intracranial aneurysms with the Enterprise stent for the diagnosis of recanalization and in-stent stenosis.
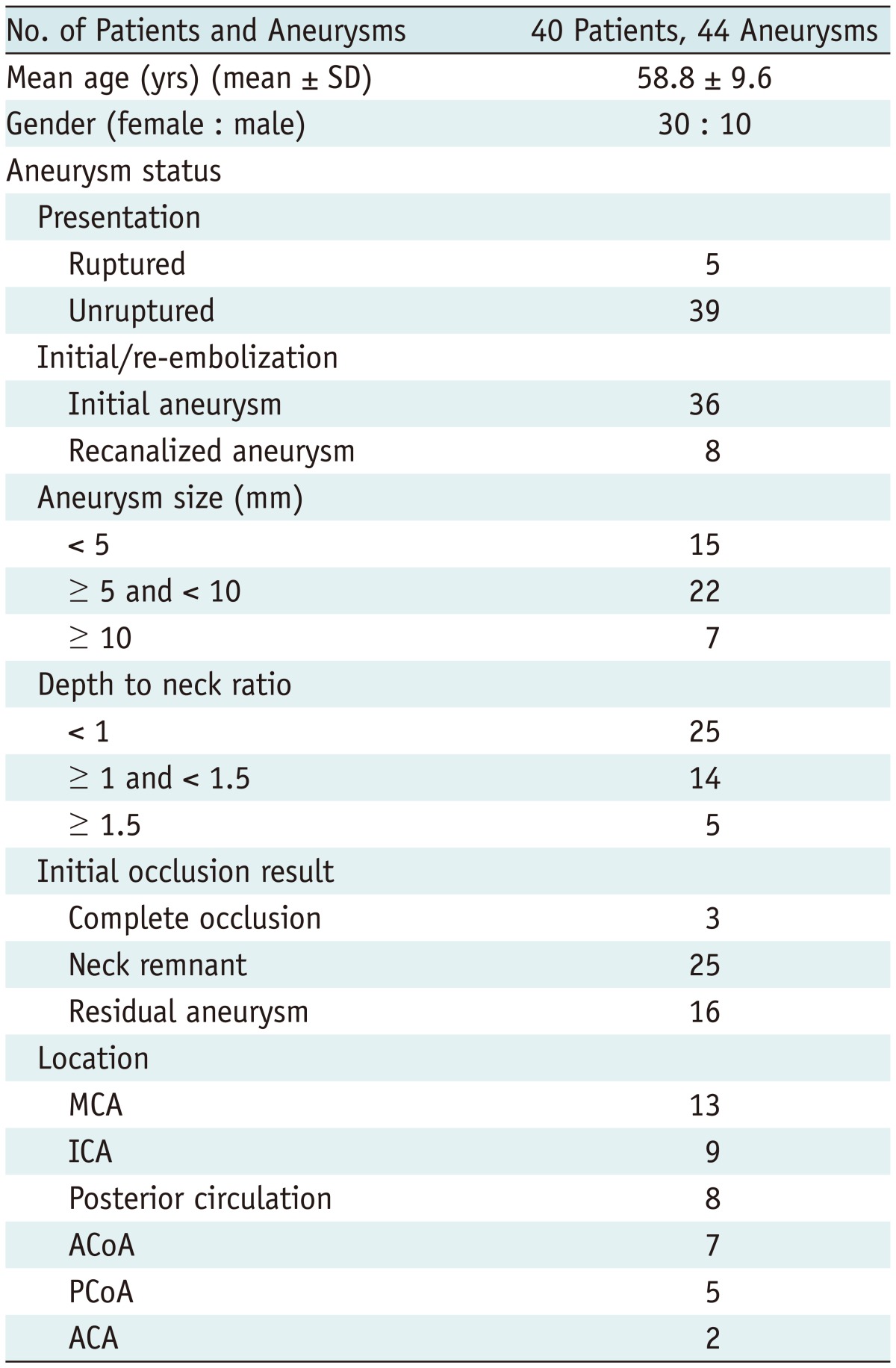
This study was conducted with the approval of the Institutional Review Board of the Seoul National University Hospital. From June 2008 to April 2012, we performed endovascular treatment for 1254 patients with 1510 intracranial aneurysms. During this period, a total of 385 aneurysms underwent coil embolization using the Enterprise stent. Among patients who had a follow-up evaluation interval of greater than 6 months, 40 patients (with 44 aneurysms) who underwent both TOF MRA and DSA follow-up with an interval gap of less than 2 months were included in this study. Clinical and demographic data of the patient population are summarized in Table 1. Our patients included 10 males and 30 females with a mean age of 58.8 ± 9.6 years. The middle cerebral artery was the most common location, followed by the internal carotid artery and posterior circulation. Most aneurysms were unruptured and 8 recanalized aneurysms were included. The maximal aneurysm diameter ranged from 1.9 mm to 23.9 mm (mean, 7.7 ± 4.5 mm). Initial occlusion immediately after coil embolization was classified using the 3-point Raymond scale (7), and 16 residual aneurysms were included. The mean time gap between the TOF MRA and DSA was 41.0 ± 25.2 days, and the interval between the follow-up DSA and coil embolization was 14.2 ± 25.2 months (median 12 months).
1.5 tesla (T) (n = 28) or 3T (n = 16) MR scanners were used for the acquisition of the 3D TOF MRA images. Intracranial 3D TOF MRA was performed in conjunction with the conventional MRI, using a multiple overlapped and acquisition technique. In the imaging with the 3D TOF MRA, the following ranges of parameters were used: 3D fast imaging with steady procession; TR, 21-38 ms; TE, 2.4-7.2 ms; flip angle, 18-25 degrees; effective section thickness, 0.5-1.2 mm; FOV, 130-190 × 130-220 (maximum intensity projection [MIP] images) and 175-240 × 190-270 (source images [SI]); and 256-640 × 192-512 matrix covering an area from the clivus to the genu of the corpus callosum. Both rotational MIP images and SI (axial acquired partitions) were used in the evaluation of the follow-up results.
The DSA was performed using a biplane system (Integris Allura; Philips Medical Systems, Best, the Netherlands) via transfemoral catheterization and selective injection of contrast media into the carotid or vertebral arteries. Imaging was performed to a standard and with adequate working projections as required.
On both the follow-up TOF MRA and DSA, the degree of coiled aneurysmal stability was classified as follows: stable occlusion (no flow or filling of the contrast within the aneurysm); minor recanalization (slight residual flow or contrast filling at the neck of the aneurysm); or major recanalization (residual flow or contrast filling of the aneurysmal sac) (Fig. 1) (8). Major recanalization required further treatment. In-stent stenosis was confirmed in the follow-up DSA. The degree of stenosis was represented as the ratio of the stented segment diameter to the diameter of the adjacent proximal non-stented segment. An in-stent stenosis of more than 33% on the DSA was considered significant (9), regardless of the presence of hemodynamic alterations or potential clinical implications.
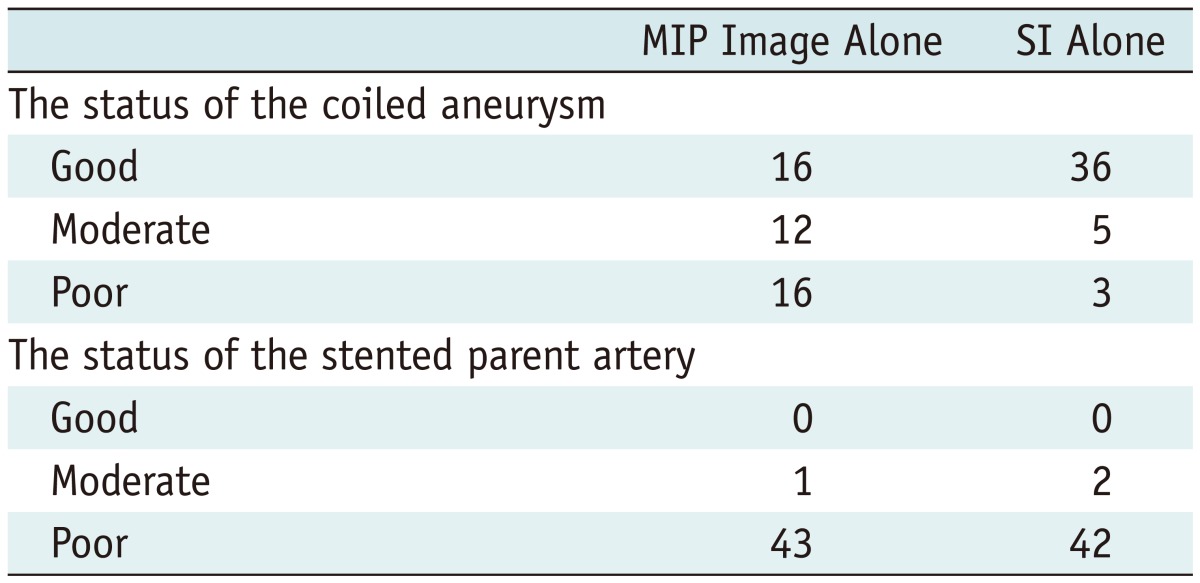
The set of follow-up TOF MRA images were randomly arranged and two experienced neurointerventionists (YDC, HSK) who were blinded to the pertinent clinical and radiologic information independently reviewed the follow-up MRA MIP images alone ('MIP mode'), and both MIP images and SIs ('SI mode') 4 weeks later to evaluate recanalization. In cases of disagreement, a consensus was established by a third interventional neuroradiologist (MHH). In addition, we compared the capability of the TOF MRA (MIP images alone and SI alone, respectively) to depict the residual flow within the coiled aneurysms and the stented parent arteries with that of the DSA (gold standard). The MR images were classified into three categories: "good" (when the images demonstrated similar features to the DSA), "poor" (when an evaluation could not be made), and "moderate" (when an evaluation could be made but information was lacking compared with the DSA) (5). Two experienced neurointerventionists (YDC, MHH) reviewed the images. Consensus was obtained in cases of a discrepancy.
The level of interobserver and intermodality agreement for the evaluation of the TOF MRA was analyzed by means of weighted kappa statistics. A comparison between the TOF MRA and the DSA for the detection of aneurysmal recanalization was made. The interpretation of κ was as follows: κ < 0 indicated no agreement; κ = 0 to 0.19, poor agreement; κ = 0.20 to 0.39, fair agreement; κ = 0.40 to 0.59, moderate agreement; κ = 0.60 to 0.79, substantial agreement; and κ = 0.80 to 1.00, almost perfect agreement. p values of < 0.05 were considered significant. All analyses were performed using Medcalc software (version 12; Mariakerke, Belgium).
Among 44 aneurysms, stable occlusion was demonstrated in 25, minor recanalization in 8, and major recanalization in 11. In-stent stenosis of more than 33% was observed in 5 patients.
In MIP and SI modes, interobserver agreement was found to be good for TOF MRA (κ = 0.835, range 0.709 to 0.961; κ = 0.731, range 0.563 to 0.898, respectively). There were 9 cases of interobserver discrepancy in the SI mode. The discrepancy between observers was caused by the following: the confusion between the normal branch and the recanalized portions due to signal loss induced by the stent (n = 5), measurement differences in the degree of recurrence (n = 2), and the difference of interpretation in the cases of small recanalized portions that were parallel to the axial acquisition of the stented artery (n = 2).
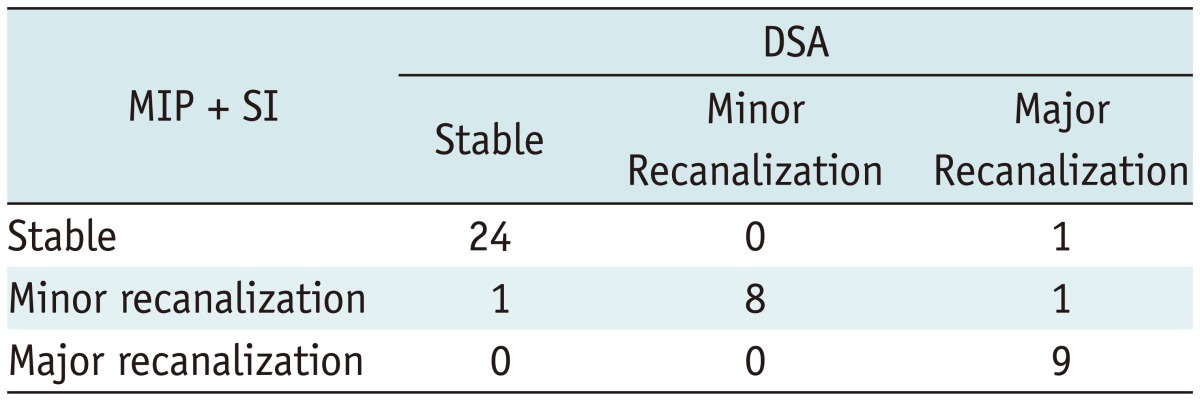
When we compared the TOF MRA with the DSA, the SI mode demonstrated almost perfect agreement (κ = 0.892, range 0.767 to 1.000), which was better than the MIP mode (κ = 0.598, range 0.370 to 0.826) (Fig. 2). There was no significant difference between the 1.5T (26/28, 92.8%) and 3T (15/16, 93.8%) MR scanners. There were 3 cases of mismatched interpretation (Table 2). Two major recanalizations on the DSA were rated as a stable occlusion in one and as a minor recanalization in the other on the MRA. The recanalized portions of both aneurysms were small and located at the paraclinoid internal carotid artery with an inferior direction. The small recanalized parts were parallel to the axial acquisition of the stented parent artery, and there were signal losses related to the Enterprise stents in the MIP images, and overlap of the recurred portion of the aneurysm with the stented parent arteries in the SI (Fig. 3). The other case of stable occlusion on the DSA was interpreted as a minor recanalization on the MRA. The discrepancy was due to suboptimal projection of the coiled aneurysm on the DSA, which hindered the revealing of a small recanalized portion covered with coils.
In terms of the depiction of the internal and neck portions of the coiled aneurysms on the TOF MRA, the SI produced better depictions than the MIP image; the rate of good or moderate depiction was 93.1% and 63.6%, respectively. With regard to the in-stent stenosis, both images showed poor depiction in most cases (97.7% and 95.5%) (Table 3).
After coil embolization for an intracranial aneurysm, a follow-up evaluation is important due to the possibility of re-opening during the follow-up period. DSA is considered the gold standard of follow-up imaging modalities (2, 6, 10). However, DSA is an invasive study and requires exposure to radiation and contrast media. MRA is a good alternative option to DSA (2, 11). There have been reports showing the good diagnostic performance of the TOF MRA in the follow-up of patients with coiled aneurysms (11, 12). Advances in stent devices make it possible to treat aneurysms with increasingly difficult configurations. Recently, the usage of stents has been increasing. In the follow-up evaluations, the stent-induced signal loss makes it difficult to evaluate the status of the aneurysm occlusion and stented parent artery (2, 4, 6, 13). Notably, our results demonstrate that the TOF MRA could be accurate and effective as a follow-up imaging modality to estimate the status of aneurysm occlusion when we pay attention to the source images. Although 3D reconstruction of MIP images had more limitations due to signal loss, axial source imaging contributed to the improved accuracy by observation of the residual flow within the coiled aneurysms. This means that the abluminal signal loss related to the stent would be less than the luminal one, and signal masking outside of the stent would not be significant enough to hinder evaluation of the re-opening of the coiled aneurysms. Therefore, we suggest that close observation of the source images in addition to the MIP images is mandatory to investigate the recanalization.
Schaafsma et al. (12) reported that a small residual lumen and suboptimal projection at the DSA were independently associated with a discrepancy between the DSA and TOF MRA. Agid et al. (14) insisted that the helmet-style remnants yielded a higher preference of the MRA compared with the DSA in the assessment of recanalization of the coiled aneurysms. In our series, we also observed a similar case. In addition, we found that it was hard to discriminate the recanalized portion from the stented parent arterial lumen if the recanalized parts were placed in a superior or inferior direction (as opposed to a medial or lateral direction), even with the axial source image analysis.
Signal loss at the stented parent artery in the MRA is caused by a susceptibility artifact and radiofrequency shielding (4, 13). Stent-induced MRA signal loss is known to be variable, depending on the thickness and cell design of the stent, raw materials used to make the stent, stented arterial tortuosity and the MR parameter (4-6). Neuroform stents produce less signal loss than Enterprise stents (6). The significant artifact related to the Enterprise stent may be induced by the closed cell design and tantalum markers on both ends of the stent (5). There have been many reports that Enterprise stents have lower procedural complication rates than Neuroform stents due to easy navigation and delivery (15, 16). Our study showed that the TOF MRA was sufficient to assess re-opening of the coiled aneurysms, but it was not sufficient to evaluate in-stent stenosis of the parent artery, which is problematic due to the significant chance of in-stent stenosis. Mocco et al. (17) reported that significant in-stent stenosis (> 50% based on diameter) developed in 3% of the patients treated with the Enterprise stent. According to our data, there was a 12.7% chance of in-stent stenosis (more than a 33% luminal loss), as demonstrated by DSA (9). Recently, there have been some reports that contrast-enhanced (CE) MRA had better image quality of the stented parent arteries than the TOF MRA (2, 4, 5, 18, 19). They also insisted that, especially in large residual aneurysms, the sensitivity of the CE MRA might be superior to the TOF MRA. Still, however, the CE MRA does not seem to replace DSA in the investigation of stented artery status. The limited spatial resolution and the potential for venous enhancement are limiting factors of this imaging modality. In addition, CE MRA carries a small but definite risk of nephrogenic systemic fibrosis (12). Because in-stent stenosis should be evaluated during the follow-up period in patients with intracranial stents and CE MRA cannot exactly reflect in-stent stenosis of the stented parent artery, we suggest that the TOF MRA is sufficient and effective to evaluate the aneurysms treated using Enterprise stents and that the DSA should be performed at least once during the follow-up period.
There are some limitations that should be acknowledged in this retrospective study. Both TOF MRA and DSA were not performed on the same day in most cases. Therefore, MRA might not reflect the exact condition of the aneurysms and stented parent arteries compared to the DSA. Another potential limitation is that the MRA images had a variety of different image qualities. This was due to MRA scanning using different machines in one institution and these different machines happened to be of different field strengths. These limitations also may have reflected the reality of the MRA readings in a tertiary referral hospital. In the end, we could not compare the TOF MRA and DSA with the CE MRA because the CE MRA was not included in the follow-up MR protocol of the coiled aneurysms.
This Study On The Interobserver And Intermodality Agreement Demonstrated That The Tof Mra Provided Sufficient Accuracy In The Screening For Aneurysm Recurrence After Enterprise Stent-assisted Coil Embolization, Especially With The Evaluation Of The Si, In Addition To Mip Images In The Tof Mra.
References
1. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002; 360:1267–1274. PMID: 12414200.

2. Lavoie P, Gariépy JL, Milot G, Jodoin S, Bédard F, Trottier F, et al. Residual flow after cerebral aneurysm coil occlusion: diagnostic accuracy of MR angiography. Stroke. 2012; 43:740–746. PMID: 22267824.
3. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010; 41:110–115. PMID: 19959540.
4. Choi JW, Roh HG, Moon WJ, Kim NR, Moon SG, Kang CH, et al. Time-resolved 3D contrast-enhanced MRA on 3.0T: a non-invasive follow-up technique after stent-assisted coil embolization of the intracranial aneurysm. Korean J Radiol. 2011; 12:662–670. PMID: 22043147.

5. Takayama K, Taoka T, Nakagawa H, Myouchin K, Wada T, Sakamoto M, et al. Usefulness of contrast-enhanced magnetic resonance angiography for follow-up of coil embolization with the enterprise stent for cerebral aneurysms. J Comput Assist Tomogr. 2011; 35:568–572. PMID: 21926851.

6. Choi JW, Roh HG, Moon WJ, Chun YI, Kang CH. Optimization of MR Parameters of 3D TOF-MRA for Various Intracranial Stents at 3.0T MRI. Neurointervention. 2011; 6:71–77. PMID: 22125752.

7. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.

8. Kang HS, Han MH, Kwon BJ, Kwon OK, Kim SH, Choi SH, et al. Short-term outcome of intracranial aneurysms treated with polyglycolic acid/lactide copolymer-coated coils compared to historical controls treated with bare platinum coils: a single-center experience. AJNR Am J Neuroradiol. 2005; 26:1921–1928. PMID: 16155135.
9. Lee SJ, Cho YD, Kang HS, Kim JE, Han MH. Coil embolization using the self-expandable closed-cell stent for intracranial saccular aneurysm: a single-center experience of 289 consecutive aneurysms. Clin Radiol. 2013; 68:256–263. PMID: 23017739.

10. Kovács A, Möhlenbruch M, Hadizadeh DR, Seifert M, Greschus S, Clusmann H, et al. Noninvasive imaging after stent-assisted coiling of intracranial aneurysms: comparison of 3-T magnetic resonance imaging and 64-row multidetector computed tomography--a pilot study. J Comput Assist Tomogr. 2011; 35:573–582. PMID: 21926852.
11. Pierot L, Portefaix C, Boulin A, Gauvrit JY. Follow-up of coiled intracranial aneurysms: comparison of 3D time-of-flight and contrast-enhanced magnetic resonance angiography at 3T in a large, prospective series. Eur Radiol. 2012; 22:2255–2263. PMID: 22569997.

12. Schaafsma JD, Velthuis BK, Majoie CB, van den Berg R, Brouwer PA, Barkhof F, et al. Intracranial aneurysms treated with coil placement: test characteristics of follow-up MR angiography--multicenter study. Radiology. 2010; 256:209–218. PMID: 20505063.

13. Plank CM, Wolf F, Langenberger H, Weber M, Beitzke D, Stadler A, et al. Improved detection of in-stent restenosis by blood pool agent-enhanced, high-resolution, steady-state magnetic resonance angiography. Eur Radiol. 2011; 21:2158–2165. PMID: 21556908.

14. Agid R, Willinsky RA, Lee SK, Terbrugge KG, Farb RI. Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced MR angiography versus catheter digital subtraction angiography. AJNR Am J Neuroradiol. 2008; 29:1570–1574. PMID: 18499789.

15. Higashida RT, Halbach VV, Dowd CF, Juravsky L, Meagher S. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol. 2005; 26:1751–1756. PMID: 16091525.
16. Weber W, Bendszus M, Kis B, Boulanger T, Solymosi L, Kühne D. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. Neuroradiology. 2007; 49:555–561. PMID: 17476494.

17. Mocco J, Fargen KM, Albuquerque FC, Bendok BR, Boulos AS, Carpenter JS, et al. Delayed thrombosis or stenosis following enterprise-assisted stent-coiling: is it safe? Midterm results of the interstate collaboration of enterprise stent coiling. Neurosurgery. 2011; 69:908–913. discussion 913-914. PMID: 21670718.

18. Gauvrit JY, Leclerc X, Caron S, Taschner CA, Lejeune JP, Pruvo JP. Intracranial aneurysms treated with Guglielmi detachable coils: imaging follow-up with contrast-enhanced MR angiography. Stroke. 2006; 37:1033–1037. PMID: 16528000.
19. Seok JH, Choi HS, Jung SL, Ahn KJ, Kim MJ, Shin YS, et al. Artificial luminal narrowing on contrast-enhanced magnetic resonance angiograms on an occasion of stent-assisted coiling of intracranial aneurysm: in vitro comparison using two different stents with variable imaging parameters. Korean J Radiol. 2012; 13:550–556. PMID: 22977321.
Fig. 1
Examples of angiographic interpretation.
Degree of coiled aneurysmal stability was classified as stable occlusion (A), minor recanalization (B), and major recanalization (C).

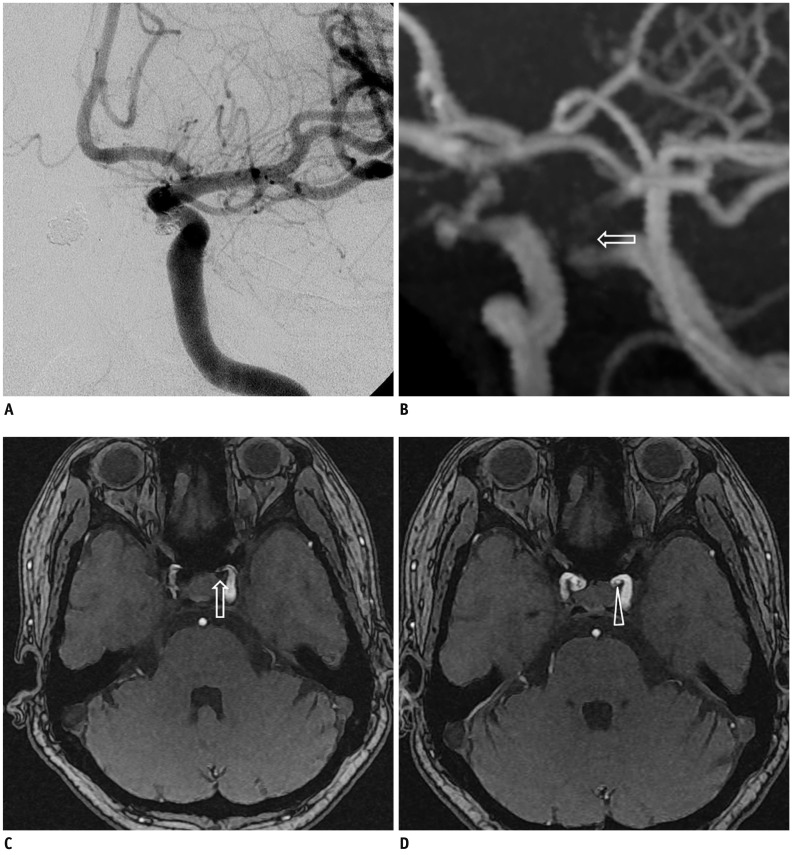
Fig. 2
Exemplary case of major recanalization.
A. Cerebral angiography after coil embolization using Enterprise stent for paraclinoid internal carotid artery aneurysms showed neck remnant with small size. B. In TOF MRA performed at 6 month follow-up examination, MIP image did not show any flow within coiled aneurysm (arrow indicates stented parent artery). C. However, some flow within aneurysmal sac was suspected in source image (arrowhead indicates recanalized portion). D. Conventional angiography showed aneurysm to be visibly recanalized. TOF = time-of-flight, MRA = MR angiography
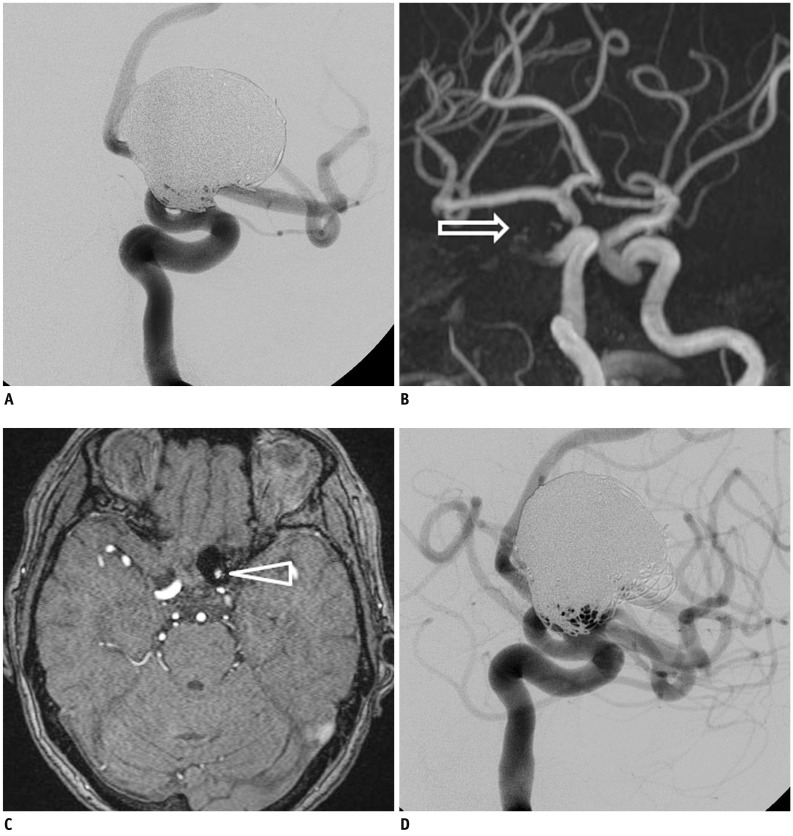
Fig. 3
Exemplary case of mismatched interpretation.
One-year follow-up angiography showed major recanalization of coiled aneurysm in distal internal carotid artery (A). However, MIP (B) and source image (C) of TOF MRA failed to show any flow within aneurysm (arrows indicate coiled aneurysm). This was in contrast to source image of pre-embolization MRA (D) (arrowhead indicates untreated aneurysm). TOF = time-of-flight, MRA = MR angiography, MIP = maximal intensity projection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download