This article has been corrected. See "Erratum: A Newly Designed Y-shaped Covered Stent in the Palliative Treatment of Hepatic Hilar Malignant Obstruction: Case Report" in Volume 14 on page 390.
Abstract
We report a case in an inoperable patient with the hilar malignant biliary obstruction treated palliatively by the use of a newly designed Y-shaped covered stent without interfering contra-lateral bile duct. We percutaneously inserted a newly designed Y-shaped covered stent into a biliary tree in an inoperable patient with Bismuth Type II cholangiocarcinoma. We checked tubograms, enhanced CT studies, and blood bilirubin levels before, one week after, and at every three month after the stenting, by observing closely the signs of clinical infection as well. The follow-up period was about 12 months. The placement of the Y-shaped covered stent was successful and resulted in adequate biliary drainage in the immediate post-procedural tubogram and in the follow-up abdominal CT. The serum bilirubin levels did not show elevation after the insertion of the Y-shaped covered stent.
The resectable rates for the hepatic hilar malignant obstruction are variable depending on surgeons, but generally reported to be approximately 10-20% (1-3). Therefore some patients are treated palliatively rather than curatively. Bilateral biliary drainage with stenting for hilar malignant obstructions has been performed for palliative treatment (4, 5). Primary patency rate with the current biliary stents in 12 months has been reported as approximately 12-40% (1, 4, 5). When the stent is obstructed by the tumor ingrowth or sludge formation, restenting can be reasonable and an ideal option to preserve the functional volume of liver in the course of chemotherapy or to prevent the bile-stasis cholangitis. However, the necessity of biliary drainage of both liver lobes is still controversial in the hilar malignant obstruction, including the advanced Klastkin tumor (6). It is difficult to re-stent bilaterally in order to drain bile, because the wires of the previously inserted stent have a potential risk of preventing the additional stent insertion through the contra-lateral bile duct (7). For this reason, there was a need for a special stent system to improve the patency rate and to allow re-stenting bilaterally when reobstruction happens in patients with a hepatic hilar malignancy.
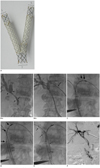
We introduce a new attempt to improve the patency rate of the biliary stent in patients with inoperable cholnagiocarcinomas by the insertion of a newly designed Y-shaped branched covered stent. It consists of two different stents: a main piece of stent and a contra-lateral limb covered stent (Fig. 1A).
A 72-year-old woman was admitted to the hospital complaining of right upper quadrant pain. Initial abdominopelvic CT showed hepatic hilar obstruction due to cholangiocarcinoma, extending from hilum of extrahepatic bile duct to distal common bile duct. Elevated total bilirubin level (13.0 mg/dL) and direct bilirubin level (10.8 mg/dL) were found at admission. Alkaline phosphatase level (1189 IU/L) and gamma-glutamyl transpeptidase (229 mg/dL) were also elevated. Percutaneous transhepatic biliary drainage (PTBD) through the right intrahepatic bile duct (Fig. 1B-a) resulted from a Bismuth type II cholangiocarcinoma. We planned on the newly designed Y-shaped covered stent insertion on the fourth day after the initial drainage procedure, and performed another PTBD through the left intrahepatic bile duct (S3 duct) (Fig. 1B-b).
Before inserting the Y-shaped biliary covered stent, the drainage catheters were removed and 8 Fr sheaths inserted over a 0.035-inch hydrophilic guide wire (Terumo, Tokyo, Japan) bilaterally. First, we inserted and located the main piece of stent into the common bile duct through left PTBD tract (Fig. 1C). The proximal end of the main piece stent (EGIS KEY-MB stent) (S&G Bio, Seoul, Korea) was located in the left intrahepatic bile duct (IHD) beyond the boundary of the hilar tumor (Fig. 1C, thick arrows). The short limb covered portion of the main piece stent was directed toward the left side of the common bile duct (Fig. 1C, thin arrows). To easily insert and deploy the contra-lateral long limb covered stent (EGIS KEY-CL stent) (S&G Bio, Seoul, Korea) without kinking its struts, the long limb covered stent of the main piece was not to be deployed (Fig. 1D, three thick arrows) until the contra-lateral long limb covered stent was completely deployed in an adequate position. After successful negotiation of a guide wire into the short limb covered stent of the main piece, a contra-lateral long limb covered stent was inserted through the right PTBD tract to connect this short limb covered stent of the main piece under the guidance of a 0.035-inch guide wire. After the confirmation that its proximal end was located in the right IHD beyond the boundary of the hilar tumor, we deployed the contra-lateral long limb covered (Fig. 1E). Finally, the long limb covered stent of the main piece was completely deployed. A 7 Fr drainage catheter was placed through the left IHD with its tip located in the common bile duct. To confirm the correct stent expansion and function, post-stenting cholangiography was performed through the drainage catheter just after the stent insertion (Fig. 1F) and 7 days later. Cholangiography showed good bilateral communication via completely expanded stents, and the drainage catheter was removed. On eighth day following the stent placement, total bilirubin and direct bilirubin levels decreased to 2.7 mg/dL and 2.2 mg/dL, respectively. They have not been elevated in the 12 months following the Y-shaped covered stent insertion (total bilirubin/direct bilirubin = 0.9/0.5 mg/dL).
In hilar malignant biliary obstruction, the first requirement for the ideal stent platform is complete coverage of the tumorous bile ducts without interfering with contra-lateral or branched bile duct patency. Gwon et al. (2) reported that percutaneous Y-configurated covered biliary stent placement for the malignant hilar obstruction gives the patent biliary flows without interfering the contra-lateral bile flows. However, even if internal biliary drainage with the other currently available biliary stent is optimally implanted in case of the bilateral hilar malignant obstructed lesion, it may actually impair the contra-lateral bile flow. This is from the wires of the second stent partially left inside the first stent in the hepatic hilum, when T-configured or Y-configured current stent is inserted. Naitoh et al. (8) cited that, in case of the biliary stent-in-stent deployment, even in T or Y configuration, there is a risk of its preventing the bile inflow in the area of the stent overlap, leading to sludge formation. Furthermore, there is a possibility that tumor ingrowth will easily occur if the stent mesh is expanded in the area of overlap. On the other hand, the present newly designed Y-shaped covered biliary stent may provide a higher patency rate, as it is a covered stent system. It does not interfere with the contra-lateral bile flow with its stent-by-stent deployment and its structural configuration (Fig. 1A). If complete coverage of the branched bile ducts with the biliary covered stent is achieved, early reobstruction occurs, and the primary patency rate of the biliary stent will be lower. This is from the wires of the previously inserted stent having a potential risk of preventing the additional stent insertion through the contra-lateral bile duct, when the reobstruction happens (3, 8). This Y-shaped covered biliary stent may not prevent the additional stent insertion, even if the biliary obstruction happens again.
From the technical perspective, the methods used in this Y-configured covered stent implantation are relatively simple, other than at one point of the procedure. Two guide wires are separately inserted into the common bile duct portion through the bilateral tumorous bile ducts. After the adequate main piece stent system (EGIS KEY-MB stent) deployment, the contra-lateral limb covered stent (EGIS KEY-CL stent) is inserted into the short limb covered portion of the main piece stent system. There is one technical step which is quite different from the usual stenting procedure. During the deployment of the main piece stent system, the operator should not deploy the long limb covered portion of the main piece system (Fig. 1E). If the operator deploys the long limb covered portion of the main piece system completely, it may interrupt the negotiation of the guide wire or long limb covered stent-introducer passing through the hilar tumorous obstructive lesion into the short limb covered stent of the main piece system.
Stent grafts or covered stent are usually more likely to prevent tumor ingrowth than bare stents. Gwon reported that the stent graft may prolong the patency rate in the hilar malignant obstruction (2, 4). However, there are few limitations in the use of a stent graft or covered stent for treating the advanced hilar bile duct malignancies. There is a risk of occlusion of branching or contra-lateral bile ducts in a covered stent or stent graft. The other possible limitation is the potential risk of migration. Hence, the partially covered stents were made with bare ends, which provides better anchoring and stabilization. In this Y-covered stent design, bare stent extensions on each end of the covered stents (main trunk, ipsilateral proximal limb, and contra-lateral limbs) will limit the chances of migration (Fig. 1A). In this case, a silicone-covered self-expandable stent system was used, because it provides a larger lumen for bile flow and does not allow as much sludge build-up or occlusion as the polyethylene-covered stent (9).
Currently there have been only a few stent trials performed for the palliative treatment of the hepatic hilar malignant obstruction (1, 4, 5), and the image appearances of the hilar lesions have seemed to be satisfactory. However, long-term or mid-term clinical outcome studies, including the occurrence of reobstruction after the stent insertions, are still sparse. In fact additional therapies including the radiotherapy, photodynamic therapy, and chemotherapy can lead to longer symptomatic survival, or to longer phases of stent patency after the conservative biliary drainage (7, 10). It is hoped that this new stent design may give clinicians the opportunity to offer a long-term patent rate, with its covered stent inserted by the Y-shaped stent-by-stent deployment.
In conclusion, the newly designed Y-shaped covered stent placement for hilar malignancies provides the following advantages: its ability to drain the bile ducts of both lobes of liver; and the suppression of the tumor ingrowth effectively, by using silicon covered stents without structural occlusion of contra-lateral bile ducts. However there is a need for further clinical studies to confirm its long term patency and the ease of the restenting technique.
Figures and Tables
Fig. 1
New stant and its use for hepatic hilar malignant obstruction treatmeant.
A. Newly designed Y-shaped Covered Biliary Stent. Y-configured stent appearance can be achieved by connecting short limb covered stent of main piece and contra-lateral long limb covered stent. Diameter of Y-shaped branched covered stent is 10 mm at main bare trunk (T) and 7 mm at covered limb stents (L).
Insertion of Y-shaped covered biliary stent in 72-year old woman with type II cholangiocarcinoma. B-a. Initial cholangiogram showed patient's tumor as Bismuth type II cholangiocarcinoma (Arrows indicates cancerous proximal and distal margins). B-b. For insertion of newly designed Y-shaped covered stent, we made another percutaneous transhepatic biliary drainage (PTBD) tract through left IHD and inserted drainage catheters (arrows) bilaterally. C. After bilateral PTBD, we inserted and located main piece of stent into common bile duct through left PTBD tract, under guidance of 0.035-inch guide wire. Proximal end (thick arrows) of main piece stent (EGIS KEY-MB stent) was located in left intrahepatic bile duct (IHD) beyond boundary of hilar tumor. Short limb covered portion of main piece stent was directed toward left side in common bile duct (Thin arrows indicate lateral markers of short limb covered stent). D. Only short limb covered stent (thin arrows) of main piece was deployed, and long limb covered stent (thick arrows) of main piece (EGIS KEY-MB stent) was not deployed. E. After confirmation of its proximal end located in right IHD beyond boundary of hilar tumor and distal end located within lumen of short limb covered stent, we deployed contra-lateral long limb covered stent (arrows) (EGIS KEY-CL stent) (S&G Bio, Seoul, Korea). F. Post-stenting cholangiography showed good internal drainage into duodenum and bilateral IHD communication, after completely deploying long limb covered stent of main piece. Outer diameter of Y-shaped covered stent introducer is 7 Fr.
References
1. Bae JI, Park AW, Choi SJ, Kim HP, Lee SJ, Park YM, et al. Crisscross-configured dual stent placement for trisectoral drainage in patients with advanced biliary hilar malignancies. J Vasc Interv Radiol. 2008. 19:1614–1619.
2. Gwon DI, Ko GY, Yoon HK, Kim YJ, Kim TH, Lee WH, et al. Safety and efficacy of percutaneous Y-configured covered stent placement for malignant hilar biliary obstruction: a prospective, pilot study. J Vasc Interv Radiol. 2012. 23:528–534.
3. Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol. 2011. 46:326–332.
4. Gwon DI, Ko GY, Yoon HK, Kim JH, Lee JM, Ohm JY, et al. Prospective evaluation of a newly designed T-configured stent graft system for palliative treatment of advanced hilar malignant biliary obstructions. J Vasc Interv Radiol. 2010. 21:1410–1418.
5. Kim CW, Park AW, Won JW, Kim S, Lee JW, Lee SH. T-configured dual stent placement in malignant biliary hilar duct obstructions with a newly designed stent. J Vasc Interv Radiol. 2004. 15:713–717.
6. Inal M, Akgül E, Aksungur E, Seydaoğu G. Percutaneous placement of biliary metallic stents in patients with malignant hilar obstruction: unilobar versus bilobar drainage. J Vasc Interv Radiol. 2003. 14:1409–1416.
7. Golfieri R, Giampalma E, Fusco F, Galuppi A, Faccioli L, Galaverni C, et al. Unresectable hilar cholangiocarcinoma: multimodality treatment with percutaneous and intraluminal plus external radiotherapy. J Chemother. 2004. 16:Suppl 5. 55–57.
8. Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009. 24:552–557.
9. Tsang TK, Pollack J, Chodash HB. Silicone-covered metal stents: an in vitro evaluation for biofilm formation and patency. Dig Dis Sci. 1999. 44:1780–1785.
10. Mihalache F, Tantau M, Diaconu B, Acalovschi M. Survival and quality of life of cholangiocarcinoma patients: a prospective study over a 4 year period. J Gastrointestin Liver Dis. 2010. 19:285–290.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download