Abstract
Desmoplastic fibroma is a rare benign primary bone tumor that is histologically similar to the soft tissue desmoid tumor. It most often involves the mandible, large long bone or iliac bone. Desmoplastic fibroma in a toe has been extremely rarely reported. Authors report a rare case of desmoplastic fibroma of bone occurring in the distal phalanx of a foot, with descriptions of the radiographic and MRI findings, correlation of the radiologic and pathologic findings, and discussion on the differential diagnosis of the tumor.
Desmoplastic fibroma is a rare benign primary fibrous bone tumor that histologically and biologically resembles soft tissue desmoid tumor (1, 2). The tumor most often involves the mandible, large long bone or iliac bone (2). Desmoplastic fibroma in the distal phalanx of toe has been very rarely been reported. Furthermore, there is relatively little description of the radiology and MRI findings of the lesions in those reports (3). We report a case of desmoplastic fibroma occurring in the distal phalanx of foot, describe the radiographic and MR findings, correlate the radiologic and pathologic findings, and discuss on the differential diagnosis of the tumor. Because of its locally aggressive nature with the high recurrent rate of the tumor, the recognition of this entity is important for the proper management for the lesion (4). This article will be helpful to establish the differential diagnosis in the slow growing osteolytic lesions affecting the distal phalanx of foot, including desmoplastic fibroma of bone.
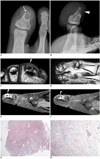
A 14-year-old male was presented with a several month history of intermittent painful swelling in the first toe. A clinical examination showed that the first toe was swollen, painful and tender to palpate. There was no specific medical or family history. Forefoot radiographs revealed a well-defined, thin sclerotic margined, oval, osteolytic lesion in the distal phalanx of the first toe. The lesion was eccentrically located within the medullary cavity. There revealed the cortical thinning and cortical breakthrough in dorsal aspect of the distal phalanx. The associated pathologic fracture through the distal portion of the osteolytic lesion was also demonstrated. There were no demonstrable matrix mineralization or distinctive periosteal reaction (Fig. 1A, B). On MRI, the well demarcated, ovalshaped, osteolytic lesion was composed of the central and peripheral parts showing different signal intensity and gadolinum contrast enhancement from each other. The central part of the mass showed low signal intensity on both T1-weighted image (T1WI) and T2-weighted image (T2WI). This central area was little contrast-enhanced on fat-suppressed T1WI after gadolinium administration. The surrounding peripheral part showed isointensity or intermediate signal intensity on T1WI, intermediate to high signal intensity on T2WI and short tau inversion recovery (STIR) image, and heterogeneous gadolinium with areas of intense enhancement, and no to minor enhancement in other areas. MRI also clearly demonstrated the cortical thinning and breakthrough in the dorsal aspect of the distal phalanx, with extension of the small soft tissue mass into the subungal area of the first toe (Fig. 1C-F). The patient underwent total excision of the lesion. Histologically, the tumor was not encapsulated, but was vaguely delineated from the non-neoplastic tissue. At low power, the tumor displayed a central hypocellular area surrounded by peripheral cellular area. At high power view, the central hypocellular area showed sclerosis with dense collagen deposition. The peripheral cellular area was composed of spindle cells forming fascicles. The tumor cells exhibited minimal cytologic atypia and no mitotic figures. On microscopic examination, no osteoid or chondroid materials were identified, essentially excluding the possibilities of osteogenic or chondrogenic bone tumors. These histopathological features were consistent with a benign fibrogenic tumor, suggesting a diagnosis of desmoplastic fibroma of bone (Fig. 1G, H).
In 1958, Jaffe first described desmoplastic fibroma of bone as a distinct entity (1). It is the benign primary fibroblastic bone tumor that may be locally aggressive. Desmoplastic fibroma histologically and biologically mimics the soft tissue desmoid tumor. Hence, desmoplastic fibroma of bone is also referred to as desmoid tumor of bone (2). It is known to be rare. The incidence of desmoplastic fibroma is reported to be 0.1-0.3% among all primary bone tumors (2). Age distribution is the highest in the first three decades of life. Clinical signs are nonspecific. Pain and swelling are the predominant symptoms, but some patients may be asymptomatic. Pathologic fracture can occur. Almost any bone can be affected, but the mandible, the femur, the tibia, and the pelvis are sites of predilection (4). The most common locations are the metaphyses and diametaphyses in long bones. It may also rarely arise in the diaphysis. Histologically, it is characterized by its collagen producing fibroblasts. These fibroblasts display a striking monotony in their spacing throughout the lesion, as well as in their cellular features. The fibroblastic cells are characteristically arranged in intertwining fascicles, or contain wavy collagen fibers loosely grouped in sweeping bundles (Fig. 1H) (4). On radiograph, desmoplastic fibroma is usually a welldefined, sclerotic or non-sclerotic margined osteolytic lesion with a narrow transition zone. The irregular trabeculated or bubbly appearance is common, and it reflects the uneven destruction and expansion of the medullary bone by a slow growing process. The cortex is usually thinned and expanded, and cortical breakthrough is not uncommon. If cortical breakthrough is present, a soft tissue mass is rarely or sometimes associated. Periosteal new bone formation is rare (4). Desmoplastic fibroma had been described that it characteristically contains no significant mineralized matrix. Callahan et al. (5), however, reported on the cartilage metaplasia in desmoplastic fibroma. In the present case, there was no chondroid or osteoid matrix within the mass, either radiographically or histologically. In contrast to radiographic appearance, MR findings of desmoplastic fibroma of bone have scarcely been described. According to the previous studies on the MR features of the tumor, desmoplastic fibroma shows isointensity or hypointensity on T1WI, isointensity or hypointensity on T2WI (in part, at least), and the heterogeneous enhancement of the mass on fat-suppressed T1WI after gadolinium administration (6, 7). Foci of isointensity or hypointensity on T2WI in desmoplastic fibroma of bone are attributed to the collagenous relatively acellular parts of the mass, analogous to its soft tissue counterpart (8). More cellular parts filled with fibroblasts or necrotic areas are responsible for higher signal intensity parts of the mass on T2WI. The heterogeneous contrast enhancement seems to reflect the heterogeneous histological composition of the tumor. It is assumed that the area with a denser cell population will undergo a greater enhancement than hypocellular and collagenous parts (8, 9). In our case, the central area of low signal intensity on both T1WI and T2WI, and little contrast enhancement represented dense foci of collagen, whereas peripheral areas of the tumor with heterogeneously high signal intensity on T2WI and STIR image, and heterogeneous contrast enhancement proved histologically to be compact cellular parts (Fig. 1G, H). The correlations between the histological and the MR features of this case seemed to be shared by those in a subset of soft tissue desmoid tumor, as previously described in the literature (8, 9). The associated pathologic fracture might affect MR signal intensity of the mass in our case. However, a good radiologic-pathologic correlation was proven in the proximal portion of the unfractured mass. When desmoplastic fibroma of bone occurs in the distal phalanx of foot, as in our patient, it should be differentiated from a variety of osteolytic lesions showing, in part, short T2 foci on MR imaging. The differential diagnosis may include intraosseous epidermal inclusion cyst, keratoacanthoma, giant cell reparative granuloma, giant cell tumor (GCT), glomus tumor and osteoid osteoma. Intraosseous epidermal inclusion cyst characteristically manifests as well defined round osteolytic lesion on radiograph. This lesion has intermediate signal intensity on both T1WI and T2WI due to their heterogeneous histopathologic components, such as multiple layers of keratin debris, dense debris in cystic centers, and intraluminal calcifications. T1WI after gadolinium administration shows thin peripheral rim enhancement without central enhancement, which may distinguish it from desmoplastic fibroma of bone (10). Keratoacanthoma is a rare, benign, rapid growing tumor located in the most distal part of the nail bed. On MR imaging, keratoacanthoma usually has intermediate signal intensity on T1WI and mixed signal intensity on T2WI. These features may be explained by the high keratin content. It also shows thin peripheral rim enhancement that is not compatible with desmoplastic fibroma of bone (10). Giant cell reparative granuloma of bone is a rare reactive process occurring predominantly in the distal phalanx of hand or foot. The lesion has low to intermediate signal intensity at both T1WI and T2WI, which is attributed to hemosiderin deposition and fibrosis (10). GCT of bone is very rare in the phalanx of the foot. MR signal characteristics of GCT are nonspecific and similar to those of giant cell reparative granuloma. Short T2 in GCT is mainly attributed to hemosiderin deposition and may be exaggerated on gradient echo sequence of MR imagings (10). Glomus tumor is a painful subcutaneous nodule which is associated with intense, pulsating pain. It originates from outside the bone, unlike desmoplastic fibroma of bone, which it may erode the underlying bone. Glomus tumor most often demonstrates very high enhancement after contrast administration, and the signal is high on T2WI (10). In osteoid osteoma, osteoid tissue is hyperintense on T2WI and is enhanced after gadolinium administration. When nidus is calcified, calcified nidus appears as a focus of low signal intensity on T1WI and T2WI (10). Desmoplastic fibroma of bone does not metastasize but is locally aggressive and prone to recur (4). Surgical resection, with a wide margin of normal tissue where possible, is the treatment of choice (4).
In summary, MR signal intensity of desmoplastic fibroma of bone seems to be nonspecific. However, when a well demarcated, predominant osteolytic lesion showing the foci of short T2 is identified in the distal phalanx of foot, desmoplastic fibroma of bone should be included in the list of differential diagnosis, although toe is unusual the location of the tumor. The clinical, radiological, and pathological correlation is helpful to diagnosis the osteolytic lesion in the distal phalanx of foot.
Figures and Tables
Fig. 1
Fourteen-year-old male with desmoplastic fibroma in first toe.
A, B. Fourteen-year-old male with intermittent painful swelling in his first toe. Anterior-posterior (A) and lateral (B) radiographs of forefoot demonstrate well-demarcated, sclerotic margined, oval, osteolytic lesion with dorsal cortical thinning and breakthrough (arrowhead) in distal phalanx of first toe. Associated pathologic fracture through distal portion of osteolytic lesion is demonstrated (arrow). There is no demonstrable matrix mineralization within tumor. C-F. MRI of desmoplastic fibroma of bone in first toe. Small ovoid central area of well-defined, osteolytic lesion shows hypointensity on all MR sequences (arrow in C-E), including axial T1WI (C), coronal T2WI (D), and sagittal STIR image (E), and shows little enhancement on sagittal fat-suppressed T1WI after gadolinium administration (F). Peripheral area of mass shows heterogeneous high signal intensity on T2WI and STIR images, and heterogeneous high to little contrast enhancement on fat-suppressed T1WI after gadolinium administration. Dorsal cortical breakthrough with small soft tissue extension (arrowheads in C, E, F) is demonstrated. T1WI = T1-weighted image, STIR = short tau inversion recovery. G, H. Photomicrographs of desmoplastic fibroma of bone. At low-power view (G), tumor displayed central hypocellular area (arrows) surrounded by peripheral cellular area (arrowheads) (hematoxylin and eosin stain, × 40). At high power view (H), peripheral area of tumor is composed of bland spindle cells with characteristic fascicular arrangement (arrows) (hematoxylin and eosin stain, × 200).
References
1. Jaffe HL. Desmoplastic fibroma and fibrosarcoma. In : Jaffe HL, editor. Tumors and tumorous conditions of the bones and joints. Philadelphia, PA: Lea and Febiger;1958. p. 298–303.
2. Fornasico V, Pritzker KPH, Bridge JA. Desmoplastic fibroma of bone. In : Fletcher CDM, Unni KK, Merten SF, editors. The World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press;2002. p. 288.
3. Armanasco P, Ali S, John L, Williamson D. A rare tumour in a rare location: desmoplastic fibroma in a toe. Foot Ankle Surg. 2011; 17:e37–e39.
4. Inwards CY, Unni KK, Beabout JW, Sim FH. Desmoplastic fibroma of bone. Cancer. 1991; 68:1978–1983.
5. Callahan KS, Eberhardt SC, Fechner RE, Cerilli LA. Desmoplastic fibroma of bone with extensive cartilaginous metaplasia. Ann Diagn Pathol. 2006; 10:343–346.
6. Vanhoenacker FM, Hauben E, De Beuckeleer LH, Willemen D, Van Marck E, De Schepper AM. Desmoplastic fibroma of bone: MRI features. Skeletal Radiol. 2000; 29:171–175.
7. Frick MA, Sundaram M, Unni KK, Inwards CY, Fabbri N, Trentani F, et al. Imaging findings in desmoplastic fibroma of bone: distinctive T2 characteristics. AJR Am J Roentgenol. 2005; 184:1762–1767.
8. Vandevenne JE, De Schepper AM, De Beuckeleer L, Van Marck E, Aparisi F, Bloem JL, et al. New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol. 1997; 7:1013–1019.
9. Sundaram M, McGuire MH, Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol. 1987; 148:1247–1250.
10. Drape JL, Le Viet D. MR imaging of the fingers. In : Stoller DW, Li AE, Bredella MA, Potter HG, Rosenberg ZS, Bencardino JT, editors. Magnetic resonance imaging in orthopedics and sports medicine. 3rd ed. Philadelphia, PA: LW & W;2007. p. 1897–1932.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download